Effect of Incubating Poor Quality Broiler Breeder Hatching Eggs
D.E. Yoho, J.R. Moyle, A.D. Swaffar and R.K. Bramwell of the University of Arkansas report on the effects of incubating poor quality broiler breeder hatching eggs on overall hatchability and hatch of fertile in the Winter 2008 issue of the Avian Advice.
Introduction
Previous research has shown that quality hatching eggs improve the likelihood of optimum hatchability as well as result in good chick quality (Yoho et al., 2008; Moyle et al., 2008). Pathogens can penetrate, contaminating the egg shell, its membranes and the embryo (Berrang et al., 1999). Improperly handled eggs can also explode contaminating the surrounding eggs in the setter. While proper sanitation of eggs can be beneficial to overall hatchability, failure to follow recommended sanitation procedures often has negative consequence on hatchability and chick quality (Funk et al., 1949, Scott and Swetnan., 1993).
Within the poultry industry it is understood that only clean and good quality broiler breeder hatching eggs should be sent to the hatchery for incubation. Breeder managers routinely discuss this topic with contract producers with varied success. However, increased production costs dictate that every possible hatching egg be sent to the hatchery and it would seem advantageous to have some practical method for dirt removal.
Producers commonly use paper towels, rags or sanding blocks to remove dirt from eggs. If the dirt is gone, then the problem should be solved, right? But, do these cleaning methods affect hatchability or chick quality?
With these questions in mind, this study was undertaken to evaluate the effect poor hatching egg selection, improper egg handling techniques and 'cleaning' procedures on hatchability, hatch of fertile and egg contamination rates.
Materials and Methods
Eight hundred forty (840) hatching eggs were obtained from the University of Arkansas broiler breeder research farm and randomly assigned to one of seven treatment groups with 120 eggs per treatment group.
The control group was correctly set clean hatching eggs, while the remaining groups included: un-touched dirty eggs, dirty eggs wiped with a wet cloth, dirty eggs sanded with an abrasive pad, checked eggs (broken shells but no broken membranes), cull eggs (misshapen eggs or double yokes) and eggs set upside down.
Eggs were incubated under common commercial incubation conditions, hatched chicks were tallied and a residue break-out analysis was performed on all unhatched eggs. Eggs were classified as contaminated if they were obviously malodorous or had noticeable bacterial contamination.
The experiment was replicated three times. Data were analyzed using JMP® statistical software comparing the means from the observations (SAS Institute, 2006). Differences were deemed to be significant at P<0.05.
Results and Discussion
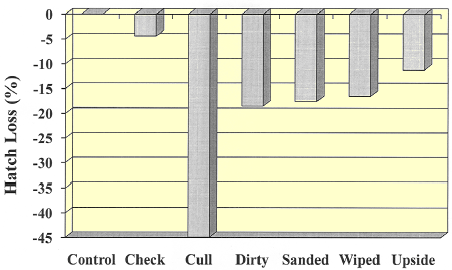
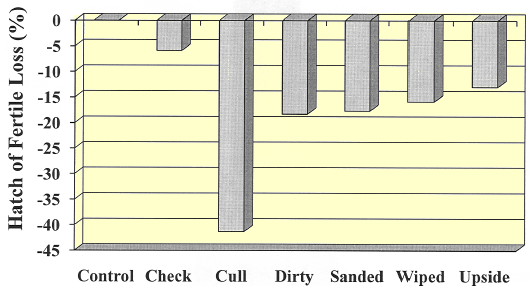
The data in Figures 1 and 2 show a significant drop compared to control in hatch and hatch of fertile in all treatment groups except checked eggs. However, the hatchability of dirty eggs that were wiped or sanded did not improve as compared to un-touched dirty eggs. Setting eggs upside down negatively affected hatchability as was expected (12 per cent) but the most significant decrease was seen in cull eggs (~45 per cent loss).
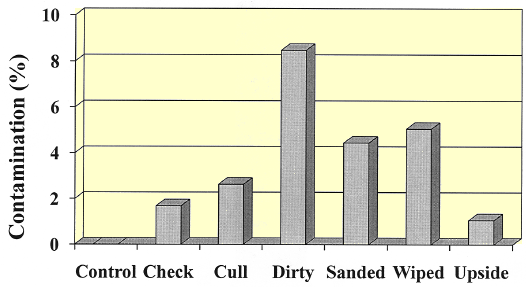
As illustrated in Figure 3, there were a significantly higher number of contaminated eggs in the dirty, sanded or wiped categories as compared to the control (8%). Once again, attempting to clean the eggs did little to improve their viability. An overall increase in exploding eggs from contamination was also observed as compared to commercial hatchery results. Exploding eggs further complicates hatchability and chick quality issues by involving the surrounding egg pack.
This experiment was an attempt to mimic the on-farm efforts to salvage dirty hatching eggs in a situation where proper sanitizing equipment may not be available. Instead, a wet rag or abrasive pad would perhaps be used.
Results indicate that there is no hatch benefit from wiping or cleaning dirty eggs. Therefore more emphasis should be placed on litter management and nest box maintenance to reduce the incidence of dirty eggs.
Conclusions
- Wiping or sanding dirty eggs does not improve hatchability.
- Setting cull eggs or setting eggs upside down will negatively affect over all hatch.
- Setting checked eggs will negatively affect over all hatch, but not to the extent first believed.
References
Berrang, M.E., N.A. Cox, J.F. Frank, and R.J. Buhr, 1999. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg. Journal of Applied Poultry Research, 8:499-504
Funk, E.M. and J.F. Forward, 1949. Effect of washing eggs on hatchability. Poultry Science, 28:155-157
Moyle, J.R., D.E. Yoho, R.S. Harper, A. D. Swaffar, R.K. Bramwell and D.J. Elfick, 2008. Egg shell color, specific gravity and hatchability, in eggs from broiler breeders. Poultry Science, 87 (Suppl.1):146
SAS Institute, 2006. SAS Institute Inc, Cary, NC.
Scott, T.A. and C. Swetnan, 1993. Screening sanitizing agents and methods of application for hatching eggs. I. Environmental and user friendliness. Journal of Applied Poultry Research, 2:1-6
Yoho D.E., J.R. Moyle, A.D. Swaffar, and R.K. Bramwell, 2008. Effect of incubating poor quality broiler breeder hatching eggs on overall hatchability and hatch of fertile. Poultry Science, 87 (Suppl.1):148