



Current Skeletal Issues in Turkeys
To remain profitable in today’s turkey meat markets, producers must grow a wholesome, competitively-priced product that exceeds the changing expectations of the consumer, according to Kenneth C. Powell, DVM at DSM Nutritional Products, Inc.Lameness causes losses for the turkey producer and strains animal welfare issues. Addressing this problem by controlling early growth with reduced lighting or feed restriction programmes helps to manage some disorders but often has negative economic rewards in live performance and meat yield. Knowing when effective preventative measures of husbandry, disease control and nutritional tools can be implemented to prevent these leg weakness/lameness losses can be important steps to the solution process and better long-term cost-effective investments for the turkey producer.
Introduction
Lameness in turkeys can range from 0-20 per cent, however due to efforts to control housing environment and disease, commercially grown turkeys within North America is quite low, often less than 3 per cent, nonetheless even a low incidence of clinical lameness at the farm can negatively impact live production parameters such as end-of-flock livability and feed conversion. “The skeleton is not only useful for carrying the bird in the turkey house, it is also important for carrying the bird through the processing plant”, was a valid point made by a USDA veterinarian, for the live production losses are only the “tip of the iceberg” compared to the economic loss this low incidence of clinical lameness has once the birds go to the processing plant. Lameness-associated downgrades and trim have a far greater economic impact adding up to several million dollars per year. According to a report by Express Markets, Inc. (Ft. Wayne, IN) on 12 April 2005, even a small reduction of 0.25 per cent in condemnations would reduce live production costs by 1/10th of a cent per pound live weight, at today’s production rate in the USA this small improvement could save the turkey producers approximately $8 million (USD) per year. Lame birds can also represent an increase food safety risk and bring higher levels of contamination into the processing plant. Thus learning to prevent and manage skeletal problems in turkeys can offer improved well-being for the birds as well as an economic reward for the producer. Proper clinical assessment and pathologic diagnosis of the skeletal lesion(s) are important for determining the cause of the lameness in a flock. Additionally, noting the timeframe which these lameness’ occur is useful for developing husbandly practices and nutritional programs that can help to prevent these diseases. For this paper, the author will attempt to cover the most common and our most pressing causes of lameness present in today’s turkey production settings, discuss ways to differentiate these lameness conditions and develop a production timeline as to when these leg weakness/lameness conditions are typically observed in a turkey flock during the grow-out lifecycle so as to aid in planning better prevention strategies.
Skeletal anatomy, composition and growth
The avian skeleton is comprised of approximately 150 bones and has two primary functions, one is to provide the framework to support muscle attachment and protect internal organs and the other function is to provide a store-house for calcium and phosphorus, two essential elements important for mineral homeostasis and metabolic processes in the bird. There are several differences between mammalian and avian skeletal structure relevant to this paper. Long bones in birds are hollow and do not contain marrow like mammalian bones; however females at sexual maturity and the onset of egg production can fill the medullary cavity with a woven bone called medullary bone that is readily mobilised during egg shell formation. The epiphysis in birds rarely ossifies. Another difference, relevant to current skeletal disorders seen today, is that the thoracic vertebrae in chickens and turkeys are fused except for joint between thoracic vertebrae, T-5 and T-6; and thoracic vertebrae T-6 and T-7. Figure 1 and 2 depict general bone anatomy relevant to discussions in this paper.
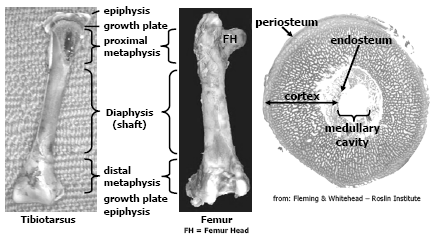
The bones are essentially comprised of a collagen/protein matrix and mineral. The collagen/protein matrix (osteoid) gives the bone its torsion and tension strength. Osteoid is a product of osteoblast (bone-forming cell) activity, these cells not only lay down this matrix but begins the mineralisation process. This immature bone, called woven bone serves to support the bird as the bone matures. Osteoid comprises about 35 per cent of the weight of a mature bone and is formed throughout the growing phase of the poult but the bulk is formed during embryonic development and the first 3-4 weeks post-hatch. As the bone matures, the osteoid becomes more organised and mineralised through the activity of osteoclasts (bone-remodeling cell), this bone is called lamellar bone. The mineral content of mature bone accounts for about 65 per cent of its weight and confers the hardness and compression strength to the bird’s bone, without it the bone would be flexible (rubbery). The mineral component is composed chiefly of calcium phosphate, about 58 per cent of the weight of the bone, calcium carbonate (7 per cent), calcium fluoride, magnesium phosphate and sodium chloride (2 per cent or less respectfully). Approximately 99 per cent of all the calcium and 80 per cent of all the phosphorus in the bird’s body is contained in the skeletal framework and these minerals are easily mobilised from the bones (through the activity of the osteoclast) when intakes or assimilation of calcium and phosphorus is deficient to meet the metabolic needs of the bird. Thus it is important to note that the mineral composition in bone is not constant and changes with age, dietary formulation and diseases particularly enteric diseases that impact digestion and absorption of fat, fatsoluble vitamins, calcium and phosphorus.
Adapted from: Banks, William J.: Chapter 9: Osteogenesis. In: Applied Veterinary Histology. p. 154. William &Wilkins, Baltimore, 1981.
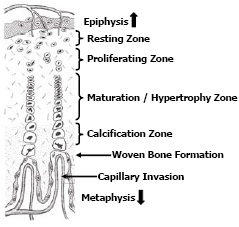
Bone growth is a dynamic process that occurs as the bird grows and to a lesser extent upon maturity. Long bones grow lengthwise (longitudinal growth) by a steady proliferation, remodeling and calcification of cartilage cells (chondrocytes) located in the growth plates (physis) found adjacent to the proximal and distal epiphyses. Some irregularly shaped long bones such as the femur may have an additional growth plate adjacent to the femur head. The growth plates appear in chickens and turkeys approximately 5-7 days post-hatch. Growth plates consist of 4-5 zones of cartilage cells in an ordered array from the epiphysis to the metaphysis termed, the zone of resting chondrocytes; the zone of proliferating chondrocytes; the zone of maturation and hypertrophy and; the zone of calcification respectfully (Figure 3).
The chondrocytes within the resting zone help integrate the growth plate to the epiphysis. The zone of proliferation contains actively dividing chondrocytes; however the maturation and hypertrophy of these cells elongate the bone. The hypertrophy zone is probably the weakest area in the growth plate for fractures in the growth plate usually occurs through this zone causing pathologies termed “epiphyseolysis” or “femur head necrosis”. Capillary invasion is an important trigger to switch chondrocytes from proliferation to hypertrophy and to calcify the matrix. Failed or poor vascular invasion into the growth plate can produce a cartilage “plug” of proliferating chondrocytes referred to as dyschondroplasia. Thus expansion of the growth plate is a concert in unison, whereby proliferation and hypertrophy of chondrocytes on the epiphyseal-side of the growth plate is balanced by capillary invasion and mineralisation to woven bone on the metaphyseal-side of the growth plate; balance is required if the growth plate is to maintain a uniform thickness (Figure 4). Longitudinal growth ceases when the growth plate thins and becomes mineralised.
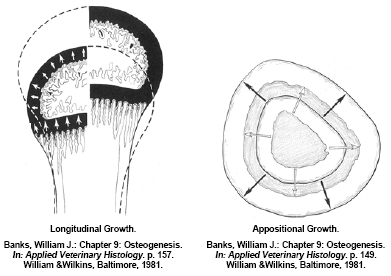
Long bones also grow in diameter (appositional growth) by remodeling the bone matrix along the bone shaft. Appositional bone growth is also a concert in unison of osteoid deposition and initial mineralisation by osteoblasts forming woven bone and paralleled osteoclast activity to remodel and further mineralise this immature bone to mature, organised lamellar bone. Much of the appositional bone growth occurs on the surface of the diaphysis with immature bone deposition accruing on the outside surface (periosteum) and the resorption/remodeling occurring on the inside surface (endosteum). In a normal bird the net effect is an expansion of the outside and inside diameter of the bone whilst maintaining the original shape and architecture (Figure 4). Appositional growth of bone is much analogous to that of the growth of a tree. Much like a forester would “read” the growth rings to evaluate historic climatic events; a good veterinary histopathologist could evaluate cross-sections of bone to determine what and when historic pathological events occurred during a turkey’s development. If osteoblast activity is affected or the substrates of osteoid are deficient during its formation, a quantitative osteoporosis can develop, in other words the bone may develop normally but there simply is not enough of it – an example would be a normal bone with a thin cortical wall of mature structural bone. If osteoclast activity is affected or there is inadequate mineral (calcium) intake, a qualitative osteoporosis develops resulting in a ricketic bone. The cross-sectional architecture of a bone is genetically determined however mechanical stress on weight-bearing bones such as the femur will modify appositional development. This architecture change can be beneficial in forming a stronger bone that is better resilient to the stress(s) that caused the response but it may make the bone for susceptible to fracture when different loads are placed on the bone (Lanyon, L.E., 1992). An example of appositional architecture change to mechanical strains might be explained in a current leg disorder seen in market age heavy tom turkeys having a spontaneous spiral fracture along the midshaft of the femur.
Skeletal Pathologies
Even though skeletal disorders in growing turkeys represent a small percentage of live production losses, they continue to be an opportunity for the industry to improve. Solutions for much of today’s abnormalities likely reside in areas of genetic selection, improved husbandry practices, disease management, particularly enteric diseases, and nutrition to include feed manufacturing, nutrient/anti-nutrient interactions as well the ever moving nutritional requirements of the modern commercial strains. It’s interesting to review the literature of the past 10-15 years covering leg abnormalities in turkeys and broilers, what was discussed then is still relevant today! It’s not that the poultry industry has not learned and applied the knowledge of the past – it surely has. Skeletal disorders and leg weakness are prevalent today simply because we are continuing our quest to minimise these problems for improved performance, economics and animal well-being with a bird that continues to improve in growth performance, feed efficiency and meat yield. If the prevalence of leg disorders is considered unsatisfactory it would simply mean that our tools for managing these conditions are out-of-step with the bird’s genetics.
There are several reviews in the literature that list and discuss most, if not all, the known skeletal abnormalities identified in poultry but to list them would be redundant and beyond the scope of this paper (Nelson, T.S., 1990; Julian, R.J., 1990; Riddell, C., 1992; Thorp, B.H., 1992; Edwards, H.M., 1992; Whitehead, C.C., 1996). Peter Woodward also offers a review of nutritional strategies that reduce leg problems in turkeys (Woodward, P., 2004). In his review he classifies nutritional-associated leg problems as those caused by mineral deficiencies, by litter related or that are growth rate related, this is an excellent way to categorize today’s leg problems for it helps the producer approach the problem from a solution orientation. To supplement this approach, I find leg problems tend to fall into three critical periods of a bird’s grow-out lifecycle. Preempted intervention with husbandry practices and added nutritional support has shown to be helpful in preventing leg problems during these critical periods.
1st Week Skeletal Defects – Maternal Deficiencies
Poults with leg problems in the 1st week are often found to have thin, pliable ricketic bones and a “beaded” appearance in joints of the ribs. Overall poult quality and mortality may also be impacted with poults having dehydration, omphalitis, and pericarditis. Though this could be a problem with brooding, early feed intakes and/or feed formulation in the affected turkey flock causing a calcium, phosphorus and/or vitamin D3 deficiency, it is more often an indication of a maternal deficiency in these nutrients. In a study by Bar et al., 1982, it took 17-21 days for poults started on a vitamin D deficient diet to demonstrate signs of rickets. Oftentimes this deficiency is not observed in the breeder hens but shows up in the offspring. To differentiate field rickets seen in older birds with ricketic bone abnormalities seen in the 1st week, certainly in the 1st 4-5 days of age, the term "congenital" rickets is sometimes used. In this sense congenital means that the poults were likely hatched with the problem. In addition to the field problem with baby poults, there may be a reported decrease in hatchability for the breeder source flock(s) with a reduction in hatch-of-fertile attributed to elevated embryonic mortality, "failure to pip" and poults with shortened mandibles (Stevens et al., 1984).
A deficiency in the breeder hen can be a result of poor early frame development during the rearing phase and/or a hot climatic environment causing anorexia and reduced nutrient intakes of calcium, phosphorus and vitamin D3 in the early lay phase. Either impacts total skeletal reserves of the hen resulting in downstream problems such as thin eggshells and low levels of 25-OH D3 in the egg for the developing embryo. Elevated embryo mortality and young poult mortality often relate to bacterial contamination of thin, porous eggshells (poor shell quality). Shell porosity also affects conductance thus altering the desiccation rate and elevating internal egg temperatures thus affecting embryonic development, hatch profiles and subsequent poult quality. Poults for the 1st week are dependent on maternal transfer for their initial D3 source, even though 25-OH D3 levels are reported to persist in the poult for up to 2 weeks post-hatch (Austic and Scott, 1997; Bar et al., 1982; Sunde, et al., 1978), the maternal levels of 25-OH D3 can vary widely. Metabolically in the embryo, 25-OH D3 is needed for modeling and remodeling of new bone as well as to help assimilate calcium for other physiological functions (namely skeletal muscle function and cellular osmolarity (Kubota et al., 1981; Hart and DeLuca, 1985). The 70-90 per cent of the calcium for the developing embryo comes from the shell (Richards and Packard, 1996). Vitamin D3 and 25-OH D3 is transferred from the hen to the egg. Low levels negatively affect hatch and elevations in 25-OH D3 improve hatch (Abdul-Rahim et al., 1979; Hart et al., 1986; Manley et al., 1977). 25-OH D3 in the egg is further metabolised by the embryo to 1,25-OH2 D3, this molecule facilitates absorption of soluble shell calcium, activates osteoclasts that remodel the embryo's bones (Haussler and Rasmussen, 1972; Lee et al., 1990) and up-regulate innate immune cells to improve early disease resistance (Swaggerty and Kogut, unpublished data, 2004).
Maternal deficiencies aside, young poults can become deficient even when the calcium, phosphorus and vitamin D3 levels are optimal in the starter diets. Turkeys hatch with a relatively immature intestinal tract (Moran, E.T., 1985). Additional reports by others describe that young poults have a limited capacity to absorb lipids, due to underdeveloped enterocyte microvilli, suboptimal brush border enzymatic functions and limited bile secretion that does not reach optimal capacity until 10-14 days of age (Stevens and Blair, 1982; Chambers and Gray, 1979; Escribano et al., 1988). This may be strain dependent, though not yet reported for the various commercial strains of turkeys, Uni, et al., 1995 and Uni, et al., 1998; report that commercial broiler strains vary as to when optimal capacity is reached. This delayed ability to absorb would only amplify any maternal deficiencies. Thus when cases of leg problems and “congenital” rickets occur in the 1st week, an evaluation of the breeder flock nutrient profile is merited.
Weeks 2-10 Skeletal Defects – Malabsorption Induced
Over the next 10 weeks skeletal frame size will increase several fold thus nutrient absorption and assimilation (fatty acids, minerals and fat-soluble vitamins) is crucial during this time of high demand. Osteoporosis seen in growing birds after the 1st week is commonly called “field” rickets. Though some skeletal defects detected in this timeframe may be a sequela to issues discussed above or a feed mixing error, more often it is the aftermath of enteric disease causing malabsorption. Early enteric insults and subsequent malabsorption causes a malassimilation of vital bone nutrients (Perry, et al., 1990). Turkeys often experience several enteric challenges that can impact proper bone formation during this period.
The first few days post-brooding can be a critical period where husbandry practices need to intervene. It is not uncommon for poults to develop litter-eating behaviors while they adjust to new surroundings and feeders; poor feeder management, lighting programmes and housing environment can exacerbate these behaviors (Schwean-Lardner, K., et al, 2006a; Schwean-Lardner, K., et al, 2006b). The subsequent enteric pathology appears 4-5 days later and metabolic bone disease (rickets) develops subsequently. Thus the post-brooding period is a critical time for it is also a time when the poults are still in a limited digestive capacity and the plasma level of 25-OH D3 from maternal contributions are waning.
Many operations in the USA will vaccinate their flocks for hemorrhagic enteritis virus (HEV) sometime between Days 18-30 with an autogenous vaccine. Some companies will boost with a second HE vaccination 1-2 weeks later. These vaccinations produce a mild enteropathy 4-5 days post-vaccination. HEV, rotovirus and turkey coronovirus are known causes of enteric disease in turkeys (Guy, J.S., 2003). Additionally other viruses (astrovirus, reovirus and torovirus) likely play a role in the pathogenesis of enteric disease complexes such as PEMS and RSS. These viral-induced damages to the intestinal epithelia can affect nutrient assimilation and absorption thus impacting feed efficiency, growth rates, flock uniformity and causing abnormal feathering, rickets and other skeletal deformities. Oftentimes these viral enteropathies predispose the intestinal epithelium to bacterial invasion by staphylococci and E. coli, leading to synovitis, osteomyelitis and mortality. Many of these viral insults occur between 2-6 weeks of age (Guy, J.S., 2006). Coccidiosis has become more recognized and prevalent in turkeys between 3-10 weeks of age. E. meleagrimitis and E. adenoeides were the most common findings in turkeys ranging from 3-5 weeks of age (Fitz-Coy, S.H., 2006). Radu reports that field studies indicate two periods of oocyst shedding, one between 5-7 weeks of age and another between 11-13 weeks of age (Radu, J., 2002). Cortes et al., 2006, reported that histomoniasis is re-emerging and responsible for mortalities as high as 50 per cent. In her latest survey, flocks diagnosed with histomoniasis ranged from 7-12 weeks of age with the highest incidence at Week 8. Intestinal roundworms (ascarids) can impact weight gains and also develop portals for secondary opportunistic pathogens such as clostridia. It is common to find roundworms in turkeys between 5-12 weeks of age. Lastly, many report a mild “flushing” in turkeys between Weeks 8-12. Its occurrence is common to the point that producers see it as a “normal” occurrence. The explanation for this diarrhea has many authors and many causes ranging from coccidia to electrolyte balance and from genetics to high levels of NSP’s in the diet – all are plausible explanations.
The mechanism for field rickets induced by malabsorption is well documented (Perry, et al., 1990; Perry, et al., 1991a; Perry, et al., 1991b; Perry, et al., 1991c; Perry, et al., 1991d; Olsen, et al.; 1981; Hedstrom, et al., Hurwitz, S., 1992). When a bird responds to any inflammatory insult, it produces a protective mucus barrier at all its mucous membranes, of particular is the intestinal surface. This protective mucus deters the absorption of fats, minerals (namely calcium) and fat-soluble vitamins. Prolonged enteric insults and subsequent fat malabsorption will deplete the poults’ labile calcium reserves, thus stimulating the breakdown of structural bone and the depletion of plasma 25-OH D3 reserves. This pathway of causation eventually leads to osteoporosis and a calcium/phosphorus/vitamin D3 deficiency within 3-5 days post-enteric insult (Figure 5).
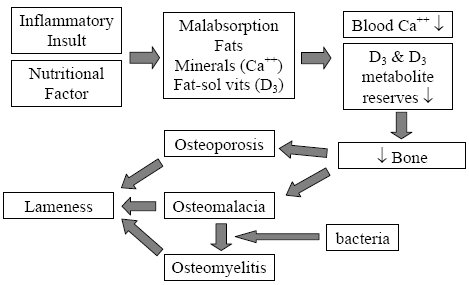
Adopted from: Skeletal Lesions Associated with Malabsorption
Perry, R; G Rowland and J Glisson AAAP Skeletal Disease Symposium, San Antonio, Texas USA 1990
Prevention of field rickets would include a review of husbandry practices, particularly the post-brooding feed management, light programmes and litter management as well as a thorough pathological work-up to best determine the time of the initial enteric insult and the causative agent(s). Nutritionists can also help reduce the skeletal damage that usually occurs as an aftermath of these early enteric insults. Field rickets can be alleviated using vitamin D metabolites, namely 25-OH D3 (Hurwitz and Bar, 1981) A current trend in turkey nutrition is to include Hy?D® (DSM Nutritional Products, Inc.), a commercial source of 25-OH D3, into turkey diets from Day 1 through Weeks 9-12 (Ward, N.E., 2003).
Weeks 10-15 Skeletal Defects – Growth-rate Induced
Enteropathy-induced field rickets could be a predisposing cause to some of the lameness and leg deformities seen in heavy tom turkeys but field observations coupled with some earlier reports suggest that it may be the result of rapid growth phase between Weeks 12- 15 or even earlier. The primary skeletal lesion, commonly seen as early as Week 12 in the field, is tibial dyschondroplasia (TD). Leach and Lilburn, 1993 provide a good review of TD mechanisms and a study by Rath, et al., 1994, gives us some insight as to the timing of TD incidence in turkeys (Figure 6).
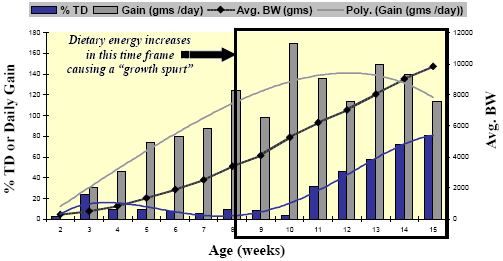
Rath, et al. Poultry Science 73:1254-1259; 1994.
Many turkeys will be able to repair these TD lesions and will not usually show clinical signs of leg weakness; however the more severe TD lesions and lesions in the heavier tom turkeys can produce clinical lameness. It is not uncommon to record that these severely lame toms will succumb to the aggressive behavior of stronger toms in the flock starting as early as Week 15. However even mild TD lesions can pose a problem for producers raising middle-weight toms (toms processed at 14-15 weeks of age) in the processing plant due to lameness-associated trim and condemnations from broken wings, breast blisters and scratches.
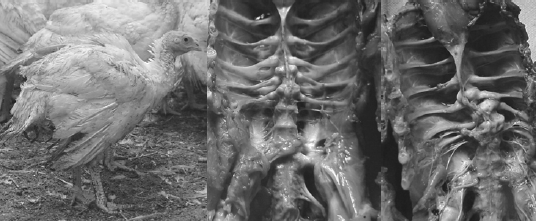
Skeletal Defects in Heavy Toms Prior to Processing
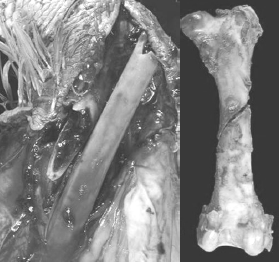
[Photo courtesy of Dr. John Barnes, NC State College of Veterinary Medicine
The author and several other colleagues have reported that as much as 50 per cent of the total flock mortality in heavy toms can occur in the last 3-5 weeks of the grow-out. Much of this late-flock mortality was due to aggressive toms on lame birds, thus attributed to having a high incidence of leg abnormalities (severe TD) at Weeks 10-15. However in another field survey on causes of late-flock mortality in tom turkeys between weeks 17 to slaughter, the author and his colleagues reported the over 30 per cent of the toms died of hemorrhage due to midshaft spiral fractures in the femur (Figure 7). This lameness impacts the animals’ well-being, production economics and grower morale.
A survey conducted by Lilburn, categorized leg abnormalities in turkeys into two major groups, TD and the other as distorted angular deformities in long bones, namely the femur due to skeletal-biomechanical imbalances (Lilburn, M.S., 1994). In his review, he reports that the femur is slower to mineralize than the tibia. A subsequent study by Applegate reports that the long bone growth in the broiler is similar whereby the mineralisation in the femur, and in particular at the diaphysis of the femur, was less than the tibia (Applegate and Lilburn, 2002). Both authors suggests that the femur may be the weak link in the skeletal frame as birds are grown to heavier body weights. However the fractures we see in the field may not be attributed to poor mineralisation of the bone as much as an architectural change in the bone as a consequence of mechanical torsions placed on the bone during development from other causes, such as an angular deformity due to dyschondroplasia of the proximal tibia or femur (Thorp, B.H., 1992). One response of bone to altered biomechanical forces is an asymmetric appositional hypertrophy of cortical bone (Figure 8).
[Photo courtesy of Dr. Doug Korver, University of Alberta]
As discussed in the section on appositional growth above, the cross-sectional architecture of a bone is genetically determined however mechanical stress on weight-bearing bones such as the femur will modify appositional development. This architecture change can be beneficial in forming a stronger bone that is better resilient to the stress(s) that caused the response but it may make the bone more susceptible to fracture when different loads are placed on the bone (Lanyon, L.E., 1992). Thus the femur may be adequately strong for standing and walking but susceptible to fracture if stressed or handled improperly when loading or transporting to the processing plant.
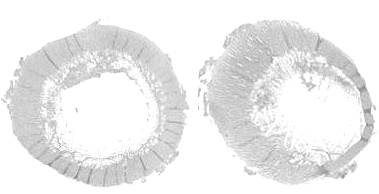
Another finding in these fractured femurs is increased cancellous bone porosity of cortex (Figure 9).
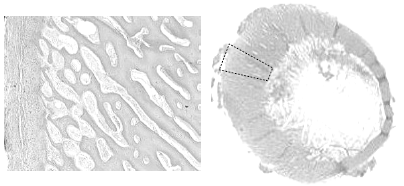
[Photo courtesy of Dr. Doug Korver, University of Alberta]
Crespo has made several observations on the porosity of fractured femurs in older breeder tom turkeys (Crespo, R., et al., 1999, 2000, 2002). In their studies the fractured femurs were significantly more porous than either the non-fractured contra lateral femur from the afflicted tom or from unaffected toms; however the mineral content of all bones was not different (Table 1).
| Table 1. Porosity differences in femurs from 32-35 week old breeder toms (Crespo, R. et al., 2002) | |||||
| Porosity | Ca % | Ca:P | Mn | Cu | |
|---|---|---|---|---|---|
| Fractured Farm A | 17.2a | 33.1b | 2.15b | 4.5ab | 0.7b |
| Non-fractured contra-lateral | 7.1b | 33.6b | 2.17ab | 4.7ab | 0.7b |
| Unaffected Farm A | 2.5b | 36.2a | 2.17ab | 5.3a | 0.7b |
| Unaffected Farm B | 6.1b | 35.4ab | 2.18a | 3.9b | 1.1a |
| a,b denote significant differences at p . 0.05 | |||||
This suggests, in addition to biomechanical torsions, that osteoclasts and/or osteoblasts have an altered cellular function thereby impacting qualitative and/or quantitative internal remodeling of the bone respectfully. This porosity may be a reflection of immaturity. This is not an uncommon finding in growing turkeys or broilers however once reaching maturity the cortical bone should become organised lamellar bone (Barnes, J.H., personal communication), however most birds seem to have immature bone to processing. Rath et al., 2000, shows an age-dependant relationship of tibial bone mineral to collagen content in broiler chickens such that older birds have a greater collagen crosslink content making the bones more resilient to breaking. Nutritional and/or genetic interventions to improve quantitative development and collagen crosslink content earlier may have merit. Remodeling is a normal process that should occur throughout the bird’s lifespan however biomechanical stress(s), the mobilisation of mineral in response to malabsorption, disuse and hormonal signals can influence this process. Thus the definitive cause(s) for this asymmetric appositional development and porosity of the femur diaphysis predisposing to fracture is still unsolved and additional funding support is needed to continue study.
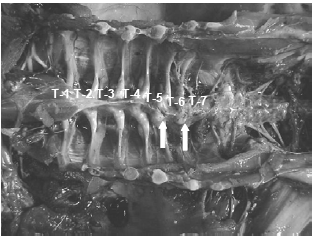
Lately the incidence of spinal deformities has increased in most all the commonly utilized commercial strains of turkeys and broilers. Birds having these spinal lesions will have a “humpback” appearance and it is not uncommon to see “shaky leg” behaviors as well and, similar to the other skeletal deformities described above, many of these weak and down birds are killed by the stronger, aggressive toms in the flock during the last few weeks before processing. The thoracic vertebrae are fused except at the joints proximal and distal of T-6, this free-moving vertebra can become ventrally (kyphosis) or laterally (scoliosis) deviated or both. It is easiest to recognise clinically in birds 14 weeks of age or older but this author has seen lesions in toms as young as 12 weeks of age (Figure 10).
The affected vertebrae at T-5 to T-7 show slight to severe deformation often to the point of impinging on the spinal cord. Osteoporosis and osteomyelitis in the body of the vertebrae is not an uncommon finding. Another finding in many birds are angular deformities in the tibia and TD. Droual et al., 1991, suggests a cause and effect between the tibial deformities and the spinal deformations as described in broilers, however the author only found the TD in less than 50 per cent of these turkey cases. The cause(s) have been attributed to genetic and developmental problems (Riddell, C., 1992; Thorp, B.H., 1992), however current opinions are that the condition is caused by a metabolic bone disease most likely a result of an earlier malabsorption condition (Young-Stamey et al., 2006).
References
Abdul-Rahim, S.M., Patel, M.B. and McGinnis, J. (1979) Effects of vitamin D3 and D3 metabolites on production parameters and hatchability of eggs. Poultry Science 58: 858- 863.
Applegate, T.J. and Lilburn, M.S. (2002) Growth of the femur and tibia of a commercial broiler line. Poultry Science 81: 1289-1294.
Austic, R.E. and Scott, M.L. (1997) Nutritional diseases. In: B.W. Calnek (Ed) Diseases of Poultry 10th edition. pp 47-73. Iowa State University Press, Ames, Iowa, USA.
Banks, William J. (1981) Osteogenesis. In: Applied Veterinary Histology. pp 138-161 Williams & Wilkins, Baltimore, USA.
Bar, A., Edelstein, S., Eisner, U., and Hurwitz, S. (1982) Cholecalciferol requirements of young turkeys under normal conditions and during recovery from rickets. Journal of Nutrition 112: 1779-1786.
Chambers, C. and Gray, R.D. (1979) Development of the structural components of the brush border in absorptive cells of the chick intestine. Cell Tissue Research 204: 387- 405.
Cortes, P.L., Crespo, R.M., Chin, R.P., Hird, D., Herve-Claude, L.P., Verdugo Vasquez, C., Charlton, B., Cooper G., Corsiglia, C., Willoughby, D. and Shivaprasad, H.L. (2006) Factors that may contribute to outbreaks of histomoniasis in commercial turkey farms in central California: a descriptive epidemiology study. Proceedings of the 55th Western Poultry Disease Conference. p 79. Sacramento, California, USA.
Crespo, R., Stover, S.M., Droual, R., Chin, R.P. and Shivaprasad, H.L. (1999) Femoral fractures in a young male turkey breeder flock. Avian Diseases 43: 150-154.
Crespo, R., Stover, S.M., Taylor, K.T., Chin, R.P. and Shivaprasad, H.L. (2000) Morphometric and mechanical properties of femora in young adult male turkeys with and without femoral fractures. Poultry Science 79: 602-608.
Crespo, R., Stover, S.M., Shivaprasad, H.L. and Chin, R.P. (2002) Microstructure and mineral content of femora in male turkeys with and without fractures. Poultry Science 81: 1184-1190.
Droual, R., Bickford, A.A. and Farver, T.B. (1991) Scoliosis and tibiotarsal deformities in broiler chickens. Avian Diseases 35: 23-30.
Edwards, H.M. (1992) Nutritional factors and leg disorders. In: C.C. Whitehead (Ed) Bone Biology and Skeletal Disorders in Poultry: Poultry Science Series Symposium 23. pp 167-193 Carfax Publishing Company, Abingdon, Oxfordshire England.
Escribano, F., Rahn, B.E. and Sell, J.L. (1988) Development of lipase activity in yolk membrane and pancreas of young turkeys. Poultry Science 67: 1089-1097.
Fitz-Coy, S.H. (2006) Turkey eimera: incidence, pathology and control. Proceedings of the 55th Western Poultry Disease Conference. pp 80-81. Sacramento, California, USA.
Guy, J.S. (2003) Turkey Coronavirus Enteritis. In: Y.M. Saif (Ed) Diseases of Poultry 11th edition. pp 300-307. Iowa State University Press, Ames, Iowa, USA.
Guy, J.S. (2006) Virus-induced enteric diseases of turkeys: lessons learned from the recent emergence of poult-enteritis mortality syndrome. Proceedings of the 2006 American College of Poultry Veterinarians and Western Poultry Disease Conference. Sacramento, California, USA.
Hart, L.E. and DeLuca, H.F. (1985) Effect of vitamin D3 metabolites on calcium and phosphorus metabolism in chick embryos. American Journal of Physiology 248: 281-285.
Hart, L.E., Schnoes, H.F. and DeLuca, H.F. (1986) Studies on the role of 1,25- dihydroxyvitamin D in chick embryonic development. Archives of Biochemistry and Biophysics 250: 426-434.
Haussler, M.R. and Rasmussen, H. (1972) The metabolism of vitamin D3 in the chick. Journal of Biological Chemistry 247: 2328-2335.
Hedstrom, O.R., Cheville, N.F. and Horst, R.L. (1986) Pathology of vitamin D deficiency in growing turkeys. Veterinary Pathology 23: 485-498.
Hurwitz, S. and Bar, A. (1981) Calcium, phosphorus and vitamin D deficiencies in young turkeys: Diagnosis and treatment. Minnesota Turkey Research. University of Minnesota Agriculture Experiment Station Reports 179: 33-41.
Hurwitz, S. (1992) The role of vitamin D in poultry bone biology. In: C.C. Whitehead (Ed) Bone Biology and Skeletal Disorders in Poultry: Poultry Science Series Symposium 23. pp 87-102 Carfax Publishing Company, Abingdon, Oxfordshire England.
Julian, R.J. (1990) Musculo-skeletal disease in poultry: an overview of current problems. Proceedings of the Avian Skeletal Disease Sypmosium, AAAP/AVMA Annual Conference. pp 1-11. San Antonio, Texas, USA.
Kubota, M., Abe, E., Shink, T. and Suda, T. (1981) Vitamin D metabolism and its possible role in the developing chick embryo. Biochemical Journal 194: 1043-1049.
Lanyon, L.E. (1992) Functional load-bearing as a controlling influence for fracture resistance in the skeleton. In: C.C. Whitehead (Ed) Bone Biology and Skeletal Disorders in Poultry: Poultry Science Series Symposium 23. pp 61-66 Carfax Publishing Company, Abingdon, Oxfordshire England.
Leach, R.M. and Lilburn, M.S. (1993) Current knowledge on the etiology of tibial dyschondroplasia in avian species. Poultry Science Reviews 4: 57-65.
Lee, S. K., Clark, N.B. and Brown, S.C. (1990) Action of 1,25-dihydroxyvitamin D3 and parathyroid hormone on 45calcium uptake by the yolk sac membrane of chick embryos. Journal of Experimental Zoology 256: 297-302.
Lilburn, M.S. (1994) Skeletal growth of commercial poultry species. Poultry Science 73: 897-903
Manley, J.M., Voitle, R.A. and Harms, R.H. (1977) Prevention of a decline in hatchability of turkey eggs by the addition of 25-hydroxy vitamin D3 to the diet. Poultry Science 56: 1352.
Moran, E.T. (1985) Digestion and absorption of carbohydrates in fowl and events through perinatal development. Journal of Nutrition 115: 665-674.
Nelson, T.S. (1990) Leg abnormalities in broilers and turkeys. Proceedings of the Pitman-Moore Nutrition for the Nineties Conference. pp 39-46. Bloomington, Minnesota, USA.
Olsen, W.G., Dziuk, H.E., Walser, M.M., Hanlon, G.F., Waibel, P.E., Stevens, J.B. and Jorgensen, N.A. (1981) Field rickets in turkey poults: Biochemical findings. Avian Diseases 25: 550-554.
Perry, R., Rowland, G. and Glisson, J. (1990) Skeletal lesions associated with malabsorption. Proceedings of the Avian Skeletal Disease Sypmosium, AAAP/AVMA Annual Conference. pp 48-58. San Antonio, Texas, USA.
Perry, R.W., Rowland, G. N., Glisson, J.R., Steffens, W.I., and Quinn, J.A. (1991a) Skeletal lesions associated with a naturally occurring poult enteritis. Avian Diseases 35: 158-164.
Perry, R.W., Rowland G. N. and Glisson J.R. (1991b) Poult malabsorption syndrome I. Malabsorption in poult enteritis. Avian Diseases 35: 685-693.
Perry, R.W., Rowland, G. N. and Glisson J.R. (1991c) Poult malabsorption syndrome II. Pathogenesis of skeletal lesions. Avian Diseases 35: 694-706.
Perry, R.W., Rowland, G. N., Foutz, T.L. and Glisson, J.R. (1991d) Poult malabsorption syndrome III. Skeletal lesions in market-age turkeys. Avian Diseases 35: 707-713.
Radu, J. (2002) Controlling turkey Coccidiosis. Proceeding of the 26th Annual North Carolina Turkey Industry Days and Poultry Supervisors’ Short Course. pp 61-64. Raleigh, North Carolina, USA.
Rath, N.C., Bayyari, G.R., Beasley, J.N., Huff, W.E. and Balog, J.M. (1994) Agerelated changes in the incidence of tibial dyschondroplasia in turkeys. Poultry Science 73: 1254-1259.
Rath, N.C., Huff, G.R., Huff, W.E. and Balog, J.M. (2000) Factors regulating bone maturity and strength in poultry. Poultry Science 79: 1024-1032.
Richards, M.P. and Packard, M.J. (1996) Mineral metabolism in Avian Embryos. In: M.A. Ottinger and M.R. Bakst (Eds) Poultry and Avian Biology Reviews, Vol. 7 No. 2/3. pp 143-161. Science and Technology Letters Middlesex, U.K.
Riddell, C. (1992) Non-infectious skeletal disorders of poultry: an overview. In: C.C. Whitehead (Ed) Bone Biology and Skeletal Disorders in Poultry: Poultry Science Series Symposium 23. pp 119-145 Carfax Publishing Company, Abingdon, Oxfordshire England.
Schwean-Lardner, K., Classen, H.L. and Fancher, B.I. (2006a) The effect of daylength on the behavior of broiler chickens. Proceedings of the 2006 Poultry Science Association Annual Meeting. abstract 204. Edmonton, Alberta, Canada.
Schwean-Lardner, K., Classen, H.L. and Fancher, B.I. (2006b) The effect of daylength on the behavior of broiler chickens. Proceedings of the 2006 Poultry Science Association Annual Meeting. abstract 205. Edmonton, Alberta, Canada.
Stevens, V.I. and Blair, R. (1982) Dietary levels of fat, calcium, and vitamins A and D3 as contributory factors to rickets in poults. Poultry Science 62: 2073-2082.
Stevens, V.I., Blair, R., Salmon, R.F. and Stevens, J.P. (1984) Effect of varying levels of dietary vitamin D3 on turkey hen egg production, fertility and hatchability, embryo mortality and incidence of embryo beak malformations. Poultry Science 63: 760-764.
Sunde, M.L., Tuck, C.M. and DeLuca, H.F. (1978) The essentiality of vitamin D metabolites for embryonic chick development. Science 200: 1067-1069.
Thorp, B.H. (1992) Abnormalities in the growth of leg bones. In: C.C. Whitehead (Ed) Bone Biology and Skeletal Disorders in Poultry: Poultry Science Series Symposium 23. pp 147-166 Carfax Publishing Company, Abingdon, Oxfordshire England.
Uni, Z., Noy, Y. and Sklan, D. (1995) Post-hatch changes in morphology and function of the small intestines in heavy- and light-strain chicks. Poultry Science 74: 1622-1629.
Uni, Z., Ganot, S. and Sklan, D. (1998) Post-hatch development of mucosal function in the broiler small intestine. Poultry Science 77: 75-82.
Ward, N.E. (2003) The use of 25-hydroxy vitamin D3 for meat poultry. Proceedings of the 30th Annual Carolina Poultry Nutrition Conference Carolina Feed Industry Association. pp 17-37. Research Triangle Park, North Carolina, USA.
Whitehead, C.C. (1996) Nutrition and bone disorder. Proceedings of the World’s Poultry Congress, Vol. II. WPSA. pp 161-171. New Delhi, India.
Woodward, P. (2004) Nutritional strategies to reduce leg problems in turkeys. Proceedings of the 31st Annual Carolina Poultry Nutrition Conference Carolina Feed Industry Association. pp 67-74. Research Triangle Park, North Carolina, USA.
Young-Stamey, S., Powell, K.C. and Barnes, J.H. (2006) Evaluation of vertebral lesions in broilers with gait abnormalities: a field study. Proceedings of the 2006 AAAP/AVMA Annual Conference. Honolulu, Hawaii, USA.











