



Strategic Use of Diagnostics
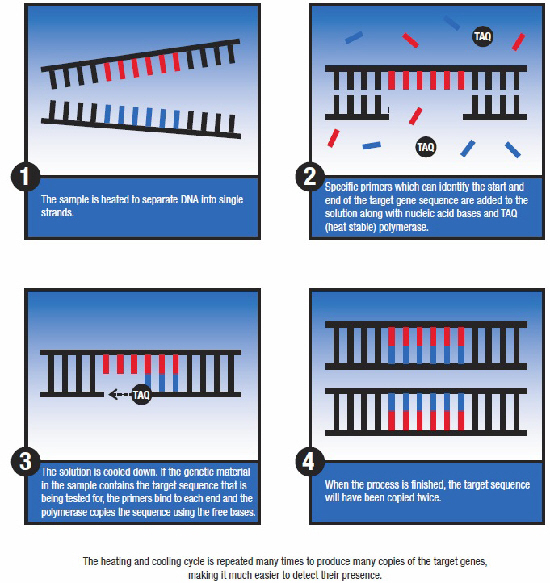
With such a wide choice of diagnostic tests available, Thermo Fisher Scientific explains how to choose the best option in any given situation.![]() Large animal veterinarians have never had such a wide range of diagnostic tools to help them maintain and improve the health and productivity of their clients’ animals. The development of new and better tests over the last few decades has made it possible to get faster, more accurate, and more precise information about the health status of individuals or groups of animals. The development of commercial ELISA (enzyme linked immunosorbent assay) and PCR (polymerase chain reaction) technologies in particular has revolutionised diagnostic services, so that veterinarians now have access to dozens of different tests covering all the major production animal species and a wide range of pathogens.
Large animal veterinarians have never had such a wide range of diagnostic tools to help them maintain and improve the health and productivity of their clients’ animals. The development of new and better tests over the last few decades has made it possible to get faster, more accurate, and more precise information about the health status of individuals or groups of animals. The development of commercial ELISA (enzyme linked immunosorbent assay) and PCR (polymerase chain reaction) technologies in particular has revolutionised diagnostic services, so that veterinarians now have access to dozens of different tests covering all the major production animal species and a wide range of pathogens.
But with such a wide choice of tests available, how do you know which one is the best option in any one given situation?
Culture, ELISA or PCR?
Unfortunately there is no simple answer, and currently no definitive or standardised guidance made available on PubMed. Although ELISA was a big step forward from the classical serology methods, and PCR is a more advanced method than the culture method, it is not simply a question of each successive development replacing the previous technology. Each has its role: even culture still has a place in the diagnostic laboratory, as Dr Gerard Wellenberg from GD Animal Health Services in The Netherlands explains:
"There will always be a need for culturing. Virology departments have shrunk as culture of many viruses has been replaced by specific PCR methods in recent years, because it is faster, cheaper and less laborious. That's why PCR was initially aimed at viruses and slow or difficult to grow bacteria. But there are still a lot of bacteria, such as Staph aureus, that can be grown in one day and are easy to grow – and, most importantly, it can still be done more cheaply than PCR."
Conversely, there are some bacteria which can now be tested for much faster and therefore more cost-effectively thanks to PCR, such as Mycobacterium avium sub-species paratuberculosis (MAP) the causative agent of Johne'-’s disease, which used to take up to six to eight weeks to culture but can be identified by PCR in a day.
"Another advantage of PCR over culture is that it can detect bacterial virulence (associated) factors and different genotypes, for example with Streptococcus suis or Haemophilus parasuis, which you can't do with culture. That makes PCR particularly useful to researchers who are investigating the epidemiology and aetiology of different pathogens."
Basic Differences between ELISA and PCR
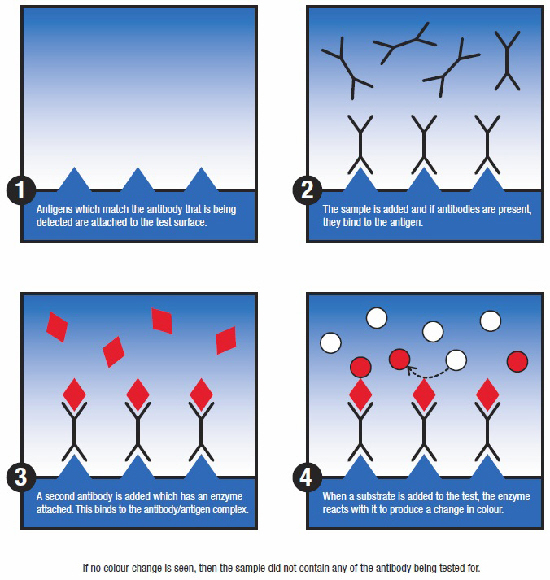
ELISA is generally used to detect antibodies and so provides indirect evidence of infection based on the animal's natural response to infection. An inherent drawback of this approach is that the presence of antibodies also could be maternally derived, or the result of a previous infection or vaccination. The latter was the driving force behind the development of so-called marker/diva vaccines with their accompanying diagnostic tools, which allow animals that have previous infection to be differentiated from those that have been vaccinated.
ELISA's for the detection of antibodies are still routinely used for surveillance and control of diseases. In the case of FMD (foot and mouth disease), the presence of antibodies to non-structural proteins by ELISA indicates that the animal was naturally infected by the virus and not positive due to vaccination.
PCR can also be developed to differentiate between field strains and vaccine strains, which can be of added value if you have a vaccination or eradication programme. Unlike most ELISA test methods, PCR is designed to detect the pathogen itself, whether a virus, bacterium, parasite, etc, and in general, it can detect the presence of a pathogen already during the incubation period, even before it produces clinical signs. However, many samples that are sent into animal health testing laboratories come from infected animals that are showing signs of disease.
There are some exceptions to the antibody/antigen differentiation between ELISA and PCR: for some pathogens, such as BVDV (Bovine Viral Diarrhoea Virus) in cattle, ELISA can also be used to detect the virus itself. However, although the basic science underlying each technology is clearly defined their usage in the field is less clear cut.
Complementary Technology
In some cases, the laboratories are asked to conduct both types of test, says Dr Wellenberg. "If the antibody ELISA is negative, customers still want to know if there is the pathogen present, because samples could have been collected during the incubation period of an infection and thus ELISA alone may not give the whole picture."
One inherent characteristic of ELISA antibody tests is that immune response takes time to develop. In the case of MAP, in some infected animals antibodies may not be detected until they are two years of age. So, the use of PCR or culture to detect MAP can be of added diagnostic value.
This synergistic use is more indicative of the way in which these tests are best viewed, he says.
"They are different systems: with ELISA you look in general for antibodies, with PCR in general you look for the agent. They are complementary. The key is to know which one to use for which pathogen."
According to Dr Wellenberg, there are a number of key parameters that need to be considered before deciding which type of test is most appropriate in a given situation.
"There is no one way of using diagnostic tools. It all depends on a whole range of factors, such as what type of programme you have: do you have a monitoring programme, or an eradication programme, or a programme for certification. All those programmes dictate how you should use your diagnostic systems.
"If you have an endemic disease or if you are virtually free of disease, then your use of diagnostic tools may be completely different."
Disease Prevalence
The prevalence of disease can be a major factor in deciding the best diagnostic approach. For example, in Holland about 70 per cent of dairy herds are sero-positive for BVDV, so it is not practical to cull all the antibody positive animals. The strategy is to identify the PI (persistently infected) animals which shed high levels of virus into the dairy herd, and then cull those individuals. It is simply not practical to test every individual animal using PCR, so bulk tank samples are tested initially to see if the herd is positive for BVDV. If it is positive, then pooled blood samples from groups of 15 animals are PCR tested to narrow down the search. Any groups that test positive are then given individual tests and the positive animals culled – for this last step, an ELISA antigen test can be used because it is cheaper than the equivalent PCR.
This strategic use of diagnostics provides a cost-effective means of identifying individual PI animals in dairy herds in a high prevalence country or area, Dr Wellenberg says.
However, in other countries which have a very low prevalence of BVDV or in countries that are BVDV-free, a totally different approach is most appropriate:
"If your country is almost BVDV free, like Sweden for example, you can aim to cull all antibody positive animals. In that case, it makes more sense to use ELISA for the initial screening because you know most animals will be antibody negative. If you get a positive result for a test, then you can use PCR to identify the individual infected animal and cull it."
Disease prevalence programmes, such as BVDV eradication in Switzerland and Germany, have been started to test all animals in order to identify all persistently infected animals via real time (RT)-PCR or ELISA antigen tests.
For monitoring programmes, for example for classical swine fever (CSF) or Salmonella in swine, ELISA tests are widely used in Holland because they are less expensive than PCR and because many laboratories have fully automated systems that can handle the large numbers of samples that are required for monitoring programmes. The ability for mass implementation and specificity always needs to be well balanced: Germany changed its testing paradigm during the last big CSF outbreaks in 2006 in pigs from antibody testing to antigen detection via RT-PCR.
The same applies at the farm level as well. A specific pathogen-free herd would take a different approach to diagnostic testing to a herd which was endemically infected with the same target pathogen. For example, a swine herd that is free of PRRS (Porcine Reproductive and Respiratory Syndrome) would take a different approach to one that has infection and sero-positive animals.
Time Window
The natural history of a disease is probably the factor that comes closest to defining the place of ELISA and PCR diagnostics. In diseased animals it generally takes seven to 14 days after infection before antibodies can be detected, so the use of ELISA is generally not appropriate during this time. Conversely, with PCR, you can get a positive result after one or two days of infection. So if you have diseased animals, many people will use PCR for identification of the causative agent.
As with most rules, there are exceptions. Schmallenberg virus for example, is only detectable in the blood of infected animals for a relatively short time, but antibody titres remain high for a relatively long time. So the use of PCR is more limited. Influenza is another example: virus particles are only present in lung and nasal swabs between day 2 to approximately day 9 – after that time, a PCR can be negative because by that stage antibodies may neutralise the virus. Again, in this situation, PCR is of limited use and ELISA may be a better option. Conversely, in PRRS infection the virus can be detected in blood up to 28 days or even longer after infection. Every animal and every agent has its own time window, and this can fundamentally affect the choice of diagnostic system used.
Sample Type
The suspected pathogen also influences the type of sample to be collected. The type of sample needed for detection is related to the tissue or cell tropism of the organism and where the organism is shed in the body during its lifecycle of infection. Swine influenza viruses, for example, cannot be detected in blood or aborted foetuses, even when the abortion is a consequence of the influenza infection. Brachyspira can be detected by faecal samples but cannot be detected in blood or lung samples.
The range of sample types that can be tested with PCR was initially quite restrictive, but more sample types are being validated for existing and new tests. ELISA, of course, can only be used on samples that will carry the antibodies or antigens that are being tested for – in most cases, that means blood.
Finances
In the current economic climate cost will always be a factor for any service linked to production animals. Diagnostic costs are a product of several factors including the resources of individual stockholders, mandatory programmes and government support, as well as the cost of individual tests or test programmes.
All these factors, and others, influence the choice of diagnostic tools and their strategic use. But, as Dr Wellenberg points out, the most costly test of all is the one that gives a misleading result because it has been applied in the wrong circumstances. Pooling, for example, is a very effective way of reducing diagnostic costs, but if your test is not sensitive enough, then pooling may provide misleading results.
There are quite a few antigen capture ELISA (ACE) which are often used as the alternative to PCR for cost reasons. Their ability to effectively replace PCR may depend on the actual viral load in the infected animals. In the case of two look-alike diseases, African Swine Fever (ASFV) and Classical Swine Fever (CSF), the titre of virus in CSF may be below the threshold of detection by ACE and thus PCR will pick up cases at the farm level that may be missed by ELISA. On the other hand, the load of viral antigens to ASFV in an infected animal are at a sufficient level that veterinary laboratories in poor countries could adequately detect ASFV by ACE if that is all that their limited resources would allow.
Many laboratories have automated ELISA systems that can handle many samples at once in an attempt to keep the cost per test down. However, PCR has become less expensive as more and more products are developed and so the cost issue is becoming less of a factor.
Talk to Your Laboratory
The large number of variables shows that it is clearly impossible to give a set of guidelines on the most appropriate diagnostic approach for each and every situation that a veterinarian might encounter in the field. Dr Wellenberg’s advice is to use the laboratories and specialized vets as a source of advice:
"Talk to your laboratory and specialized vets. Make sure you know their capabilities: what kind of tests they offer. And don’t be afraid to ask their advice – after all, they should be the diagnostic experts. They know all the latest ideas and can help you formulate the most effective test programme for your area or for individual herds."
The future is likely to see the number of diagnostic tools continue to grow: not only in the form of more PCR and ELISA systems but also the development of multiplexing and micro-chips. That means knowing which ones to use will become even more important.
For more information about diagnostics, click here.
This feature is provided by Life Technologies Corporation, which is now part of Thermo Fisher Scientific.











