Marek's disease control in broiler breeds
Author: A. Gregorio Rosales DVM, MS, PhD, DACPV - Poultry Health ConsultantIntroduction:
Marek's Disease Virus (MDV), a highly infectious and easily transmitted herpesvirus, is one of the most common viral infections worldwide and is capable of causing tumours and immunosuppression in broiler breeders. These tumours can be found in different organs of the bird, as well as in nervous, skin and eye tissue. Other viruses capable of causing tumours in parent stock are Avian Leukosis Virus (ALV) and Reticuloendoteliosis Virus (REV), which are retroviruses that can sporadically cause mixed infections with MDV, complicating their diagnosis. Occasionally tumour lesions unrelated to viral diseases can occur spontaneously in adult broiler breeder flocks.
Marek's Disease Virus continues to be a major threat for the poultry industry although in most areas it remains under control by a combination of vaccination and biosecurity practices to reduce the risk of early exposure.
Infections and Transmission
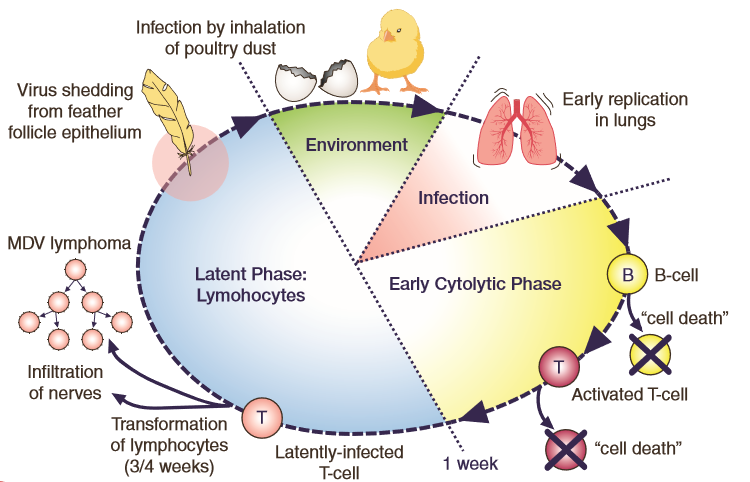
Marek's Disease Virus is highly contagious and easily transmitted from bird to bird. It replicates in the feather follicles, where it is released into the environment through dead cells of infected feather dander and persists for long periods of time. Infections occur when chicks inhale infected dander or contaminated dust and the virus reaches the lungs where respiratory tissue and blood cells (B and T type lymphocytes) become infected. B lymphocytes are responsible for humoural (antibody mediated) immunity while T lymphocytes are involved in cellular immune responses (direct defence against invading organisms.)
Eventually the virus reaches the feather follicles completing its replication cycle (Figure 1.) Effective vaccination prevents the development of tumours from latently (inactive or dormant state) infected T lymphocytes, and although infection and shedding of Marek's Disease Virus can be reduced, it cannot be fully prevented by vaccination. Infected chickens become healthy carriers for life and a source of infection to younger birds.
Figure 1. Schematic diagram showing the different stages of Marek's Disease Virus pathogenesis including the virus shedding from the feather follicle epithelium and the transformation of T lymphocytes in susceptible birds.
Marek's Disease Virus
Vaccination has been the foundation in the protection against Marek's Disease Virus. The majority of Marek's Disease Virus vaccines are cell-associated, meaning the virus is present in the cells of cell cultures, grown under laboratory conditions, and used to manufacture the vaccine. They are administered in-ovo at egg transfer (18 days of incubation) and/or at hatch by subcutaneous or intra-muscular injection.
Commercially available vaccines include the following:
1. Traditional (live virus)
- Serotype 1 - [CVI - 988 (Rispens)]
- Serotype 2 - (SB-1)
- Serotype 3 - [Herpesvirus of Turkeys (HVT)]
2. Viral-vectored recombinant HVT with gene inserts for other viruses
- rHVT - IBD
- rHVT - ND
- rHVT - ILT
- rHVT - AI
Herpesvirus of Turkeys vaccines are used in combination with serotypes 1 (Rispens) and/or 2 (SB-1) to maximise preotection in breeder flocks. Today, Herpesvirus of Turkeys combined with Rispens is the most common combination used to protect breeders in very high-risk areas.
Viral-vectored recombinant vaccines are made from viruses that have been genetically modified by using a recombinant Herpesvirus of Turkeys (rHVT) as a vector for the gene inserts (coding for immunogenic antigens) for other viruses. Examples of gene inserts that are used with rHVT vaccines include Infectious Bursal Disease (IBD), Newcastle Disease (ND), Infectious Laryngo-tracheitis (ILT) and Avian Influenza (AI). The advantage of recombinant vaccines is that they generate an immune response against both the vector and the insertion of another virus.
These rHVT vaccines should not be mixed with conventional HVT or among themselves because the response to Marek's Disease Virus and the corresponding insert will be negatively affected. Likewise, if Herpesvirus of Turkeys is used in-ovo, then rHVT cannot be used at hatch because it will result in interference against the recombinant product. Also, it is imperative to be cautious in combining Marek's Disease Virus vaccines with other kinds of live vaccines or additives (i.e antibiotics, supplements) unless specifically advised by the vaccine manufacturer. It is important to always follow the manufacturer's recommendations when administering vaccines.
Vaccine handling and preparation
Most Marek's Disease Virus vaccines are frozen in liquid nitrogen. Freeze-dried vaccine (Herpesvirus of Turkeys strain only) is available for small flocks or used in countries where liquid nitrogen is not available. Cell-associated vaccines should only be stored in specialised liquid nitrogen containers (-196 degrees centigrade), reconstituted in a specifically supplied diluent, and administered following precise procedures provided by the manufacturer. Preventing bacterial contamination during vaccine preparation and administration is key when preparing the Marek's Disease Virus vaccine. Proper mixing and hygiene procedures in the hatchery's vaccine preparation room and for the vaccination equipment must also follow the manufacturers' recommendations at all times.
Vaccine storage, thawing, reconstitution, hygiene and administration procedures require regular training of hatchery personnel and continuous auditing by internal (quarterly) and external (annually) quality control specialists.
Administering Marek's Disease Virus vaccines
The administration of Marek's Disease Virus vaccines in-ovo results in better protection against early Marek's Disease Virus challenges and has a positive effect on the chicks' immune system and responses against other non-related antigens (molecules that induce immune responses). Also, administration of Marek's Disease Virus vaccine in-ovo followed by a second dose at hatch can improve the protection against early challenge with very pathogenic strains in field situations, such as areas with a heavy concentration of poultry farms, multiple age farms, use of re-used litter and breeder chicks travelling long distances after hatch.
Reconstituted vaccines must be maintained under refrigeration while being administered and used within 30-60 minutes. Marek's Disease Virus vaccines are unstable suspensions of cells, and therefore, proper mixing and periodic shaking will prevent sedimentation and ensure a uniform does and administration.
Further information on vaccine strains, vaccination methods and vaccination regimes for breeders can be found in the Aviagen Marek's Disease booklet (2017) or by consulting with the Aviagen global veterinary services department. Selecting the most protective vaccine strain, vaccine combinations and administration methods is critical to maximise the protection against the disease and associated bird mortality.
Causes of Marek's Disease outbreaks
After vaccination in the hatchery, chicks are not protected until the Marek's Disease Virus vaccine strain multiplies in the individual chick and starts circulating in the blood (known as viremia), which can take between 4-5 days. Therefore, it is essential to reduce the risk of early environmental exposure to Marek's Disease Virus and/or delay the time of infection for as long as possible so the birds are fully protected.
Built-up litter and multiple age rearing farms pose a very high risk of early exposure to the field Marek's Disease Virus and other immunosuppressive viruses. Proper selection and administration of Marek's Disease Virus vaccines will result in adequate control; however, Marek's Disease Virus outbreaks could occur as a result of the following factors (alone or in combination):
- Inadequate vaccine storage, handling, preparation or administration procedures
- Suboptimal doses or dilution of vaccines
- Additives (antibiotics) that change the pH and/or other properties of the diluent
- Interference to Marek's Disease Virus vaccination response caused by other vaccines
- Early exposure to very virulent (vv) or very Virulent + (vv+) Marek's Disease Virus strains
- Immunosuppression caused by:
a) Infectious factors:
- Infectious Bursal Disease Virus
- Infections Chicken Anemia Virus
b) Flock management factors:
- Over heating during hatch, processing and transport
- Poor brooding conditions (incorrect house environmental conditions, inadequate provision of feed and water)
- Extreme heat and cold (environmental temperatures), inadequate ventilation
- High stocking densities, poor feed distribution, inadequate feeder space, poor body-weight development
c) Nutritional factors:
- Poor ingredient quality and mycotoxins
- Suboptimal levels of essential nutrients.
Diagnosis
Tumour like lesions can develop in birds as young as three weeks of age and typically are most common before flocks reach sexual maturity. However, tumour lesions can also be found during post-mortem examinations of flocks around peak egg production (late Marek's Disease Virus)). Since essentially all healthy birds are carriers of Marek's Disease Virus without showing symptoms, virus isolation from blood samples does not have any diagnostic value.
Diagnosis is based on typical gross lesions such as enlarged peripheral nerves, tumours in various internal organs, nodules in the feathered skin and grey, irregular eyes. It is confirmed by histological examination of a complete set of tissues (see list below) by a trained poultry pathologist.
Furthermore, a conclusive diagnosis can be achieved by histochemistry or the presence of high loads of Marek's viral DNA by polymerase chain reaction (PCR) testing. Detection of large quantities of viral DNA by real-time PCR in blood, tumour cells, and feather pulp provides a specific diagnosis of Marek's Disease Virus even in mixed infections with other viral tumour induced diseases.
A summary of required specimens and diagnostic procedures is presented below:
1) Histopathology (complete set of tissues placed in 10% buffered formalin):
- Liver, kidney, spleen, proventriculus
- Peripheral nerves (sciatic nerve), brain
- Bursa, skin, eyes
2) PCR tests and histochemistry
- Frozen tissue
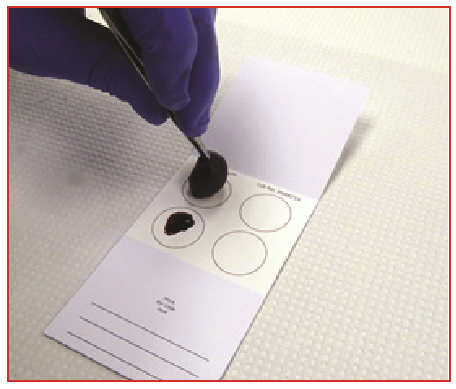
- FTA cards (imprints or tumoural tissue) (Figure 2)
- Paraffin embedded fixed tissue
Figure 2. Example of tumoural tissue imprinted onto FTA cards.
Diagnosis must be performed as a multi-step process. When mortality due to tumour lesions is suspected fresh dead, lethargic and paralysed birds must be examined at the farm or submitted to a diagnostic laboratory to collect specimens for confirmatory tests as described above. In addition, a complete flock clinical and vaccination history must be collected along with the number (or percentage) of birds with suspected tumours from the total number of birds examined.
Once the diagnosis is confirmed it will be necessary to investigate and determine the cause(s). Mixed infections with Marek's Disease Virus, ALV and REV can occur and therefore, it will be necessary to conduct various tests as histological exams alone cannot be used to make a final diagnosis. Virus isolation is only performed (from peripheral white blood cells, spleen and tumours) to evaluate the pathogenicity of a field strain.
Conclusions:
- Marek's Disease Virus is present in all commercial flocks regardless of vaccination or health status
- Marek's Disease Virus continues to be a threat due to evolving and increasingly pathogenic strains
- Marek's Disease Virus in parent stock is due to tumour lesions and immunosuppression
- Vaccination does not prevent infections and shedding of the pathogenic field virus
- Biosecurity helps to reduce risk of early exposure to Marek's Disease Virus and other immunosuppressive diseases
- Cell-associated (frozen) vaccines require careful management, and its preparation and administration require continuous training and auditing
- Careful selection of appropriate vaccine strains and vaccination methods is critical to maximising protection and should be done following manufacturer's recommendations
- Marek's Disease Virus outbreaks can be caused by improper vaccine selection, handling and administration or as a result of early exposure to highly pathogenic Marek's Disease Virus and immunosupression
- Diagnosis of Marek's Disease Virus is a multi-step procedure requiring laboratory confirmation
- If Marek's Disease Virus is confirmed, an investigation must follow to identify contributing causes.
For Further information, see the Aviagen booklet - Marek's Disease Virus and the Veterinary How to - Take FTA Card Samples found at www.aviagen.com.