



Effect of Egg Size on Heat Production and the Transition of Energy from Egg to Hatchling
By A. Lourens, Wageningen University and Research Centre, R. Molenaar, H. van den Brand, M. J. W. Heetkamp, and B. Kemp, Wageningen University, and R. Meijerhof, Hybro b.v. - An experiment was conducted to study the effect of egg size on embryo development, heat production, and energy partitioning between egg and hatchling.
Abstract
Small (56.1 ± 0.12 g SEM) and large (70.0 ± 0.11 g SEM) hatching eggs were incubated in climate respiration chambers, and eggshell temperature was maintained constant at 37.8°C in both egg weight classes by adjusting machine temperature. Dry matter, ash, protein, and fat contents were determined in albumen, yolk, yolk-free body (YFB), and residual yolk (RY), and carbohydrate contents and caloric values were calculated. To achieve a constant eggshell temperature, machine temperature needed to be set lower from d 15 onward, coinciding with increased heat production in large eggs compared with small eggs. Selective nutrient uptake resulted in higher fat content and lower protein content in RY in chicks that hatched from small eggs compared with large eggs.
The respiration quotient in small and large eggs was the same, and embryos in small and large eggs were equally efficient in the transfer of energy from egg to YFB. The surplus availability of nutrients in large eggs was therefore held responsible for the absolute and relative higher weight of RY in chicks that hatched from large eggs compared with small eggs.
INTRODUCTION
It has been reported that during incubation, large eggs produce more heat than small eggs (Rahn et al., 1974; Vleck et al., 1980; Hoyt, 1987; Vleck and Vleck, 1987; Meijerhof and van Beek, 1993). Large eggs also face more difficulties to remove the surplus heat from the egg (French, 1997), as a result of the decreasing ratio between egg surface and egg content with increasing egg size (Vogel, 1981) and the reduced air velocity over the eggs in commercial incubators (French, 1997). If large eggs and small eggs are incubated under similar conditions, the higher heat production (HP) and increased difficulties to remove heat in large eggs will result in higher embryo temperatures in these eggs (Meijerhof and van Beek, 1993, Meijerhof, 2002).
The influence of embryo temperature on embryo development and hatchability is shown by Lourens et al. (2005), who used eggshell temperature (EST) as a reflection of embryo temperature. Because egg size influences embryo temperature through heat production and heat transfer, experiments studying the effect of egg sizes on embryo development between species (Ricklefs, 1987) or late embryonic mortality within 1 species (Hagger et al., 1986; Reinhart and Moran, 1979) will be influenced by differences in embryo temperature, if incubator conditions are not adjusted to obtain an equal embryo temperature.
To our knowledge, the effect of egg size on embryo development and hatchability is never studied independent of embryo temperature. Therefore, an experiment was conducted to incubate eggs from 2 different size classes at an equal EST. The goal of the experiment was to investigate the effect of egg size on HP, embryo development, and energy transition between egg and hatchling, when eggs of different sizes were kept on the same EST of 37.8°C throughout incubation.
MATERIALS AND METHODS
Experimental Setup
In 4 trials, small and large eggs were incubated separately at a constant EST of 37.8°C in 1 of 2 identical climate respiration chambers. The EST from 5 individual fertile eggs was measured continuously, and machine temperature (MT) was adjusted automatically every 5 min if the median EST drifted away from 37.8°C. Heat production (HP) was calculated from oxygen consumption and carbon dioxide production. The amount of energy available in the egg albumen and egg yolk before incubation and the distribution of energy between the yolk-free body (YFB) and residual yolk (RY) at hatch were determined by chemical analyses.
Hatching Eggs and Incubation
First grade hatching eggs from 1 Hybro G grandparent stock were divided into 2 different weight classes: small (54.0 – 56.0 g) and large (70.0 – 72.0 g), and eggs were stored between 5 and 7 d. Per trial, 30 small eggs and 30 large eggs were incubated in 1 of 2 identical small open circuit climate respiration chambers. Additionally, from both groups, 5 eggs per trial were used to determine egg constituents.
Both climate respiration chambers (267 L) contained an automatic tray turning system that turned the eggs every 30 min at an angle of 90°. Two different fans mixed fresh air with recirculated air and provided a consistent airflow across the eggs. One temperature sensor (Pt100, Sensor Data BV, Rijswijk, The Netherlands) measured MT; a Vaisala sensor measured relative humidity. Another 5 sensors (Pt1000, Sensor Data BV) measured individual EST of 5 different eggs. Sensors were attached with tape (Tesa BV, Almere, The Netherlands) in heat conducting paste (Schaffner Holding AG, Switzerland) on the eggshell at the equator of the egg. All temperature sensors were compared after the experiments at different temperature levels between 36 and 40°C. Differences between individual sensors and the mean were maximal 0.1°C. Relative humidity was set at 55% constantly during the first 18 d.
The EST was measured every minute, and MT was automatically adjusted according the median EST of 5 different eggs to maintain EST at 37.8°C. Eggs were candled at 7 d and 18 of incubation, and infertile eggs and eggs containing dead embryos were removed. At 18 d of incubation, all eggs were reweighed to determine egg weight loss during incubation, and eggs were transferred to hatching baskets. The EST was measured again, and MT was set at the constant value that corresponded to a constant EST of 37.8°C. For the remaining time until hatching, EST was allowed to increase.
Heat Production
Oxygen and carbon dioxide concentrations were measured every 9 min in both chambers and in fresh air. Carbon dioxide concentration was measured with a nondispersive infrared CO2 analyser (type Uras 3G, Hartmann and Braun, Frankfurt, Germany). Oxygen concentration was measured with a paramagnetic oxygen analyser (type ADC7000, Analytical Development Co. Ltd., Hertfordshire, UK). The refreshed air volume was 2 L/ min during the first 18 d of incubation and 3 L/min from d 18 onward. The exact air volumes were measured with Schlumberger G1.6 dry gas meter. The HP was calculated using the formula of Romijn and Lokhorst (1961) and adjusted for fertility and embryo mortality, based on the breakout analysis described later.
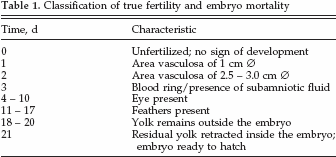
Embryo Mortality, Hatchability, and Hatchling Measurements
Clear eggs (candled at 7 d of incubation) and dead-inshell eggs (hatch debris) were opened to determine true fertility and pattern of embryonic mortality by visual appraisal. For the correction for mortality in the HP calculations, day of mortality was classified by the characteristics described in Table 1, and in more detail to the estimated day of mortality by the size of the dead embryo.
The HP was expressed per living, fertile embryo (egg) based on fertility and mortality pattern. Hatchability was expressed as the percentage of chicks that hatched from the true fertile eggs, based on the breakout analysis. At 21.5 d of incubation, all hatched chicks were killed with a mixture of CO2 and O2. All chicks were weighed, and total chick lengths were measured by stretching chicks along a ruler and taking the length between the top of the beak and the tip of the middle toe of the right feet according to Hill (2001) or Lourens et al. (2005). Next, RY was removed and weighed.
Energy Partitioning During the Transfer from Egg to Hatchling
A total of 40 fresh eggs (5 eggs per trial per egg weight class) were boiled, and albumen and yolk were separated and weighed. The eggshell was dried for 24 h at room temperature and weighed. From 40 chicks per egg weight class (5 chicks per trial per egg weight class), YFB and RY were separated for analysis. Albumen, yolk, residual yolks, and the yolk-free bodies were frozen at -18°C. At a later stage, dry matter (ISO 6496, 1983), ash (ISO 5984, 1978), protein (ISO 5983, 1998), and fat contents (ISO 6492, 1999) were determined. Carbohydrate content was estimated as 1,000 – ash – protein – fat content (grams per kilogram of DM).
Finally, the energy content in the different parts was calculated using energy densities for protein, fat, and carbohydrates of respectively 16.8, 37.8, and 16.8 MJ/kg of DM (International System of Units, 1998). The efficiency in energy transfer from available energy to YFB (EYFB) was calculated as
Statistical Analyses
The nonlinear, sigmoid curves of HP and MT were analyzed in Genstat 6.1 (2002) with a restricted maximal likelihood procedure according to the following model for repeated measurements: Yijk = . + Ei + Tj + (E x T)ij + Dk + interactions + ƒÃijk, where Yijk is HP or RQ, . is the overall mean, Ei is egg size (i = small, large), Tj is trial (j = 1,2,3,4), Dk are the 21 d of incubation (k = 1,...,21), and ƒÃijk is the residual error term. The interaction between treatment and trial (E*T)ij represents the random effect within trial between day numbers and is used as error term to test the effects of Ei and Tj.
A Generalized Linear Mixed model procedure for a binomial distribution with a logit link function (Genstat 6.1, 2002) was used for hatchability data. Egg- and chickrelated factors were analyzed by 2-way ANOVA with the GLM procedure of Genstat software (Genstat 6.1, 2002). In both procedures, a group of 30 eggs in 1 climate respiration chamber was used as experimental unit.
The model was Yij = . + Ei + Tj + (Ei ~ Tj) + ƒÃij, in which Yij is the dependent variable, . is the overall mean, Ei is egg size (i = small, large), Tj is trial (j = 1,2,3,4), and ƒÃij is the residual error term. In all analyses, nonsignificant interactions were deleted from the model. Data of DM, ash, protein, carbohydrates, and energy content were analyzed according to the latter model. Unfortunately, data of YFB and RY in trial 4 were lost, so results and conclusions with regard to energy contents and changes were based on only the first 3 trials.
RESULTS
Incubation Temperature and Heat Production
For MT, an effect of time (P < 0.001) and an interaction between time and egg size (P = 0.032) were observed. From d 15 onward, MT needed to be decreased more compared with small eggs (Figure 1). During the course of incubation, an effect of time (P < 0.001), egg size (P = 0.001), and an interaction between time and egg size (P = 0.040) on HP were observed. The HP in large eggs was higher compared with small eggs from d 15 onward (Figure 2).
Hatching Eggs, Incubation Parameters, and Hatchling Characteristics
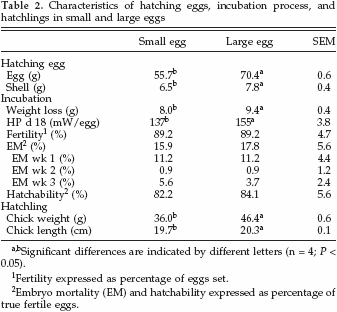
Hatching egg characteristics, incubation-related parameters, and hatchling characteristics per egg size category are summarized in Table 2. Shell weight of small eggs was lower than shell weight of large eggs, and absolute weight loss during incubation was lower in small eggs compared with large eggs. At d 18, small eggs produced 137 mW per egg (2.5 mW/g), and large eggs produced 155 mW per egg (2.2 mW/g); see Table 2. The RQ decreased between d 5 and 9 from 1.08 to 0.68, remained constant thereafter, and did not differ between small and large eggs. Embryo mortality (EM) in week 1, 2, or 3 and hatchability were similar for both egg size classes (Table 2). Chicks that hatched from small eggs weighed less and were shorter compared with chicks that hatched from large eggs.
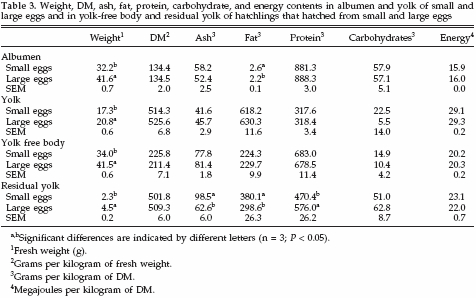
Chemical Analyses and Energy Content in Eggs and Hatchlings
Egg and hatchling constituents in both egg weight classes are shown in Table 3. Fresh albumen and yolk weights were lower in small eggs compared with large eggs. Also, YFB and RY weight were lower in chicks that hatched from small eggs compared with large eggs. Dry matter content in albumen, yolk, YFB, and RY did not differ between egg size classes. Relative ash content (grams per kilogram of DM) in RY of chicks that hatched from small eggs was higher than in RY of chicks that hatched from large eggs. The RY of chicks that hatched from small eggs also contained relatively more fat (grams per kilogram of DM) than RY of chicks that hatched from large eggs. On the contrary, RY of chicks that hatched from small eggs contained relatively less protein (grams per kilogram of DM) than RY of chicks that hatched from large eggs. No differences were observed in relative carbohydrate contents (grams per kilogram of DM) in albumen, yolk, YFB, or RY between egg size classes. Also the relative energy content (megajoules per kilogram of DM) in albumen, yolk, YFB, or RY did not differ between egg size classes (Table 3).
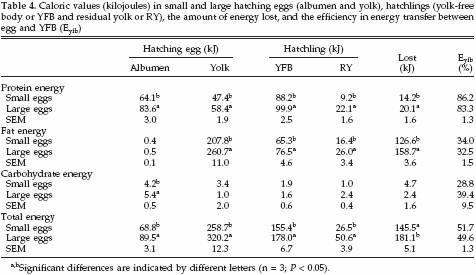
Caloric values (kilojoules in fresh product) in albumen, yolk, YFB, and RY in small and large eggs are summarized in Table 4. The total energy content in both albumen and yolk of small eggs was less than in large eggs. The total energy content of the YFB in chicks that hatched from small eggs was less than in chicks that hatched from large eggs. The total energy content of RY in chicks that hatched from small eggs was about one-half of the energy content of RY in chicks that hatched from large eggs. In albumen, the energy content of protein and carbohydrates was higher in large eggs compared with small eggs.
No differences were observed in energy contents of fats. In yolk, the energy content of protein and fat was higher in large eggs compared with small eggs, and no differences were observed in energy contents of carbohydrates. In YFB and RY, the energy content of protein and fat was higher in large eggs compared with small eggs, and no differences were observed in energy contents of carbohydrates. Also the amount of energy lost during incubation from proteins and fats was higher in large eggs compared with small eggs, and no differences were observed in amount of energy lost from carbohydrates. The relative energy distribution between albumen and yolk in small and large eggs was the same: on average 21.4% of the energy was allocated in the albumen and 78.6% in the yolk.
In both egg weight classes, on average 44.3% of the available energy in the egg was lost during incubation. The energy content of YFB and RY, however, differed between egg weight classes. Hatchlings that hatched from small eggs contained 47.5% of the available energy in YFB compared with 43.4% in large eggs (SEM = 0.6%). The RY in hatchlings that hatched from small eggs contained 8.1% of the available energy, compared with 12.3% in hatchlings that hatched from large eggs (SEM = 1.2%). The efficiency (Eyfb) of the process of transferring energy from egg to YFB was similar between small and large eggs with regard to protein, fat, carbohydrates, and total energy.
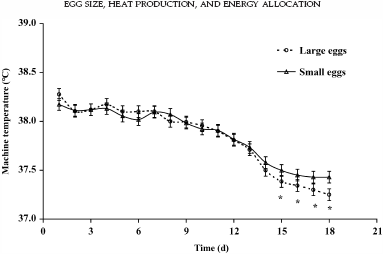 Figure 1. Machine temperature required to incubate eggs at 37.8°C eggshell temperature (EST) in small and large eggs (overall SEM = 0.06°C). |
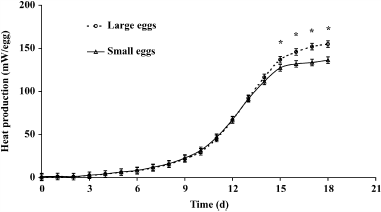 Figure 2. Heat production per egg (mW) in small and large eggs (SEM = 3.8 mW). |
DISCUSSION
Heat Production and Eggshell Temperature
From the moment that large eggs produced more heat, MT needed to be decreased more for large eggs than for small eggs. In our study we observed a higher HP and lower MT in large eggs from d 15 onward, which suggests that embryonic growth in large eggs increased more from that moment onward.
This is in accordance to the review by Wilson (1991), who concluded that embryo weight is not correlated with egg weight during the first half of the incubation period. If MT was not adjusted in the present study, EST would have increased more in large eggs than in small eggs from d 15 onward, as shown in Romijn and Lokhorst (1956) or calculated by Meijerhof and van Beek (1993). Adjusting MT avoids adverse effects of high EST during the last week of incubation on embryo development (Lourens et al., 2005).
The HP initially increases with higher temperatures (Nichelmann et al., 1998; Janke et al., 2002), but too high temperatures decrease HP during the final stages of incubation (Janke et al., 2002). The effects of high EST on embryo development and HP can also be present in the studies of, for example, Romijn and Lokhorst (1956, 1960), Tullett and Deeming (1982), Tullett and Burton (1982), Janke et al. (2004), and O’Dea et al. (2004), who incubated eggs at a constant MT of 37.8°C. In these studies, HP at 18 d of incubation ranged between 2.0 and 2.6 mW/g, comparable with the results of the present study (2.5 mW/g in small eggs and 2.2 mW/g in large eggs). Higher HP of 2.8 to 3.2 mW/g was observed by Hulet and Meijerhof (2001), who adjusted MT based on the response of the embryos on varying MT.
These high values were not confirmed in the present study, which may be due to the fact that Hulet and Meijerhof (2001) used eggs of high yielding broilers, whereas in the present study eggs of grandparents of high yielding broilers were used. Breed may influence HP, as in Tona et al. (2004), in which very large differences in HP were observed between standard heavy (4.6 mW/g) and Labeltype lines (2.7 mW/g) during incubation between 17.8 and 19.5 d for 60-g eggs. However, in the study of Tona et al. (2004), eggs were incubated at a constant MT of 37.8°C, resulting in higher EST during the final stages of incubation, accelerating the timing of internal pipping in eggs from standard heavy breeders at an earlier age than in Label-type lines. This probably had a larger effect on HP than line per se.
During the early stages of incubation, excess CO2 is being washed out from the albumen (Romanoff, 1967). From d 9 onward, the RQ value in the present trial of 0.68 indicates the oxidation of fat with additional synthesis of glycogen (Beattie, 1964; Rahn, 1981). This RQ value is lower than reported by Decuypere (1984), who measured a RQvalue of 0.72. Glycogen is stored in liver and muscles and is mobilized during the hatching process (Bell and Freeman, 1971). Wineland et al. (2000a,b) and Wineland and Christensen (2001) concluded that incubating eggs at higher MT (38.6 vs. 37.5°C) early or late in incubation resulted in less utilization of the yolk and increased utili zation of glycogen as energy source. High temperatures therefore not only reduce the required energy available for the embryo to emerge from the eggshell but also increase RQ. This may explain the differences in RQ observed between this experiment and the results of Decuypere (1984), who incubated at a constant MT and therefore increased EST at the end of the incubation process. When the embryo draws relatively more energy from the anaerobic system under high temperatures, HP and thus embryo development will be decreased.
Energy Contents and Changes
Total caloric values of eggs in the present study are comparable with data from Ar et al. (1987), who measured a caloric value of 368 kJ for an average-sized hatching egg. They reported that YFB contained on average 137 kJ and the RY 132 kJ, so on average 99 kJ was lost, which is lower than the findings in the present study. Compared with the results of Ar et al. (1987), embryos in the present study retained less energy in RY and more in YFB, produced more heat, and were therefore less efficient in energy transfer between egg and YFB. Wiley (1950) reported that embryos in small eggs find physical limitations beyond a certain size, which reduces growth and hence HP. In the present study, Eyfb was similar in small eggs and large eggs, which suggests that embryos in small eggs used the available energy equally efficiently as embryos in large eggs. Still, chicks that hatched from large eggs had a larger amount of energy left in the RY. Based on similar efficiency in energy transfer, we expect that embryos in large eggs had an surplus energy reserve that was retained unused in the RY.
To incubate small and large eggs at an equal EST of 37.8°C,MTprofiles had to be adjusted for both size classes individually to compensate for differences in HP from d 15 onward. Embryos in small and large eggs incubated at 37.8°C were equally efficient in energy transfer from egg to YFB, and the surplus of nutrients in large eggs is likely to be responsible for the larger energy content of RY in chicks that hatched from large eggs. Relative energy density was the same in small and large eggs, but selective uptake of nutrients seemed to result in a higher fat content and lower protein content of RY in chicks that hatched from small eggs compared with large eggs. Based on these results, it can be speculated that when small and large eggs are not incubated at the same EST, HP, energy utilisation, embryo development and hatchability might have been influenced more by temperature than by egg size per se. Relations between EST and nutrient uptake, growth, and HP are largely unknown and need further investigation.
ACKNOWLEDGMENTS
Hybro BV (Boxmeer, The Netherlands) kindly donated the hatching eggs. The Dutch Egg and Poultry Board contributed financially to this research. The assistance of the staff in the research facilities at Zodiac, De Haar, Wageningen, The Netherlands, was greatly appreciated.
REFERENCES
Ar, A., B. Arieli, A. Belinsky, and Y. Yom-Tov. 1987. Energy in avian eggs and hatchlings: Utilization and transfer. J. Exp. Zool. Suppl. 1:151–164.
Beattie, J. 1964. The glycogen content of skeletal muscle, liver and heart in late chicken embryos. Br. Poult. Sci. 5:285–293.
Bell, D. J., and B. M. Freeman. 1971. Physiology and biochemistry of the domestic fowl. Vol. 1. Academic Press, London, UK.
Decuypere, E. 1984. Incubation temperature in relation to postnatal performance in chickens. Arch. Exp. Vet. 38:439–449.
French, N. A. 1997. Modelling incubation temperature: The effect of incubator design, embryonic development, and egg size. Poult. Sci. 76:124–133.
Genstat 6.1. 2002. Genstat Release 6.1 Reference Manual. VSN Int., Wilkinson House, Oxford, UK.
Hagger, C., D. Steiger-Stafl, and C. Marguerat. 1986. Embryonic mortality in chicken eggs as influenced by egg weight and inbreeding. Poult. Sci. 65:812–814.
Hill, D. 2001. Chick length uniformity profiles as a field measurement of chick quality? Avian Poult. Biol. Rev. 12:188.
Hoyt, D. F. 1987. A new model of avian embryonic metabolism. J. Exp. Zool. Suppl. 1:127–138.
Hulet, R. M., and R. Meijerhof. 2001. Real time incubation temperature control and heat production of broiler eggs. Poult. Sci. 80(Suppl. 1):128.
International System of Units. 1998. BIPM, Pavillon de Breteuil, F-92312 Se`vres Cedex, France.
ISO 5983. 1998. Animal feeding stuffs. Determination of nitrogen content and calculation of crude protein content. Int. Org. Standardization (ISO), Geneva, Switzerland.
ISO 5984. 1978. Animal feeding stuffs. Determination of ash content. Int. Org. Standardization (ISO), Geneva, Switzerland.
ISO 6496. 1983. Animal feeding stuffs. Determination of moisture content. Int. Org. Standardization (ISO), Geneva, Switzerland.
ISO 6492. 1999. Animal feeding stuffs. Determination of fat content. Int. Org. Standardization (ISO), Geneva, Switzerland.
Janke, O., and B. Tzschentke, and M.L. Boerjan. 2004. Heat production and body temperature in embryos of modern chicken breeds. Proc. XXII World’s Poult. Congr., Istanbul, Turkey.
Janke, O., B. Tzschentke, J. Ho¨ chel, and M. Nichelmann. 2002. Metabolic responses of chicken and muscovy duck embryos to high incubation temperatures. Comp. Biochem. and Phys. Part A 131:741–750.
Lourens, A., H. van den Brand, R. Meijerhof, and B. Kemp. 2005. Effect of eggshell temperature during incubation on embryo development, hatchability and post-hatch development. Poult. Sci. 84:914–920.
Meijerhof, R. 2002. Design and operation of commercial incubators. In Practical Aspects of Commercial Incubation. Ratite Conf. Books, Lincolnshire, UK.
Meijerhof, R., and G. van Beek. 1993. Mathematical modeling of temperature and moisture loss of hatching eggs. J. Theor. Biol. 165:27–41.
Nichelmann, M., A. Burmeister, O. Janke, J. Ho¨ chel, and B. Tzschentke. 1998. Avian embryonic thermoregulation: role of Q10 in interpretation of endothermic reactions. J. Therm. Biol. 23,6:369–376.
O’Dea, E. E., G. M. Fasenko, J. J. R. Feddes, F. E. Robinson, J. C. Segura, C. A. Ouellette, and J. H. van Middelkoop. 2004. Investigating the eggshell conductance and embryonic metabolism of modern and unselected domestic avian genetic strains at two flock ages. Poult. Sci. 83:2059–2070.
Rahn, H. 1981. Gas exchange of avian eggs with special reference to turkey eggs. Poult. Sci. 60:1971–1980.
Rahn, H., C. V. Paganelli, and A. Ar. 1974. The avian egg: Air-cell gas tension, metabolism and incubation time. Resp. Physiol. 22:297–309.
Reinhart, B. S., and E. T. Moran. 1979. Incubation characteristics of eggs from older Small White Turkeys with emphasis on the effects due to egg weight. Poult. Sci. 58:1599–1605.
Ricklefs, R. E. 1987. Comparative analysis of avian embryonic growth. J. Exp. Zool. Suppl. 1:309–323.
Romanoff, A. L. 1967. Biochemistry of the avian embryo. John Wiley & Sons, New York, NY.
Romijn, C., and W. Lokhorst. 1956. The caloric equilibrium of the chicken embryo. Poult. Sci. 35:829–834.
Romijn, C., and W. Lokhorst. 1960. Foetal heat production in the fowl. J. Physiol. 150:239–249.
Romijn, C., and M. W. Lokhorst. 1961. Some aspects of energy metabolism in birds. In Proceedings Second Symposium on Energy Metabolism. Methods and results of experiments with animals. Eur. Assoc. Anim. Prod. Publ. 10:49–59. Brouwer, E. and A. J. H. van Es, ed. EAAP, Wageningen, The Netherlands.
Tona, K., O. M. Onagbesan, Y. Jego, B. Kamers, E. Decuypere, and V. Bruggeman. 2004. Comparison of embryo physiological parameters during incubation, chick quality, and growth performance of three lines of broiler breeders differing in genetic composition and growth rate. Poult. Sci. 83:507–513.
Tullett, S. G., and F. G. Burton. 1982. Factors affecting the weight and water status of the chick at hatch. Br. Poult. Sci. 23:361–369.
Tullett, S. G., and D. C. Deeming. 1982. The relationship between eggshell porosity and oxygen consumption of the embryo in the domestic fowl. Comp. Biochem. Phys. 72A:529–533.
Vleck, C. M., D. Vleck, and D. F. Hoyt. 1980. Patterns of metabolism and growth in avian embryos. Am. Zool. 20:405–416.
Vleck, C. M., and D. Vleck. 1987. Metabolism and energetics of avian embryos. J. Exp. Zool. Suppl. 1:111–125.
Vogel, S. 1981. Life in moving fluids. Princeton Univ. Press, Princeton, NJ.
Wiley, W. H. 1950. The influence of egg weight on the prehatching and post-hatching growth rate in the fowl. II. Egg weight-chick weight ratios. Poult. Sci. 29:595–604.
Wilson, H. R. 1991. Interrelationships of egg size, chick size, post-hatching growth and hatchability. World’s Poult. Sci. J. 47:5–20.
Wineland, M. J., and V. L. Christensen. 2001. Impact of hatchery conditions on chicks. In Completed Research. Dec. 2001. US Poult. Egg Assoc.
Wineland, M. J., K. M. Mann, B. D. Fairchild, and V. L. Christensen. 2000a. Effect of high and low incubator temperatures at different stages of development upon the broiler embryo. Abstr. 180 in Int. Poult. Sci. Forum. SPSS, Tucker, GA.
Wineland, M. J., K. M. Mann, B. D. Fairchild, and V. L. Chistensen. 2000b. Effect of different setter and hatcher temperatures upon the broiler embryo. Abstr. 181 in Int. Poult. Sci. Forum. SPSS, Tucker, GA.
September 2006











