



Soy oligosaccharides and beta-conglycinin, behind gut inflammations, wet droppings and footpad dermatitis in chickens
In this article the impact of soy oligosaccharides and the antigen beta-conglycinin on the productive performance and intestinal health of broiler chickens is reviewed.Soy oligosaccharides; behind footpad dermatitis
Stachyose and raffinose, key constituents of the soy oligosaccharides, are not successfully digested in the monogastric intestinal tract due to the absence of endogenous α-1,6-galactosidase activity in the intestinal mucosa (Gitzelmann and Auricchio, 1965). The poorly digested carbohydrates exert an osmotic effect in the duodenum and jejunum portion of the intestinal tract until they are fermented in the cecal tonsils (Marteau and Boutron-RUault, 2002). Higher osmotic pressure delineating from consumption of noxious quantities of soy oligosaccharides creates abnormally aqueous digesta that increases the feed passage rate in broilers (Coussement, 1999; David and Peter, 2001).. Jiang et al. (2006) demonstrated a negative effect of increasing dietary levels of stachyose on the performance of broiler chickens (Figures 4 and 5).
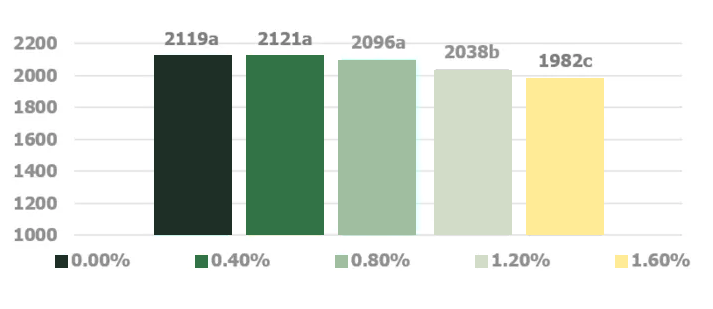
SED = 17.5
Significant linear effect
a-b Values with different subscript are significantly different (p<0.05) (Figure adapted from Jiang et al. 2006)
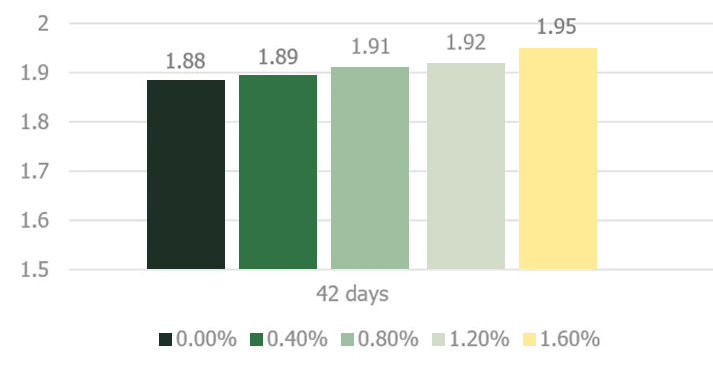
SED = 0.01
Significant linear effect
Figure adapted from Jiang et al. 2006
Jiang et al. (2006) utilized an enzymatically processed soybean meal low in soy oligosaccharides as their primary protein source as a design to effectively demonstrate the negative impacts of stachyose (figure 4 and 5). Stachyose content in practical diet formulations can exceed 6% of the soybean meal fraction as confirmed by Perryman et al. 2013 and García-Rebollar et al. (2016). In a common broiler starter diet that contain 30% SBM inclusion, stachyose content can reach 1.8% which exceeds the highest levels that elicited a negative response in Jiang et al. (2006). As previously mentioned, diets comprising high concentrations of soy oligosaccharides have the capacity to induce wet fecal droppings which can spawn wet litter and footpad dermatitis. Perryman et al. (2013) reported a decrease in the incidence of footpad dermatitis when reducing the content of soy oligosaccharides in broiler diets through the use modified soybean meal (Figure 6). Taken together these findings support the negative impact soy oligosaccharides may exert on live performance and footpad quality. Strategies which help limit, remove, or reduce the oligosaccharide fraction in SBM may help ameliorate the previous described performance maladies.
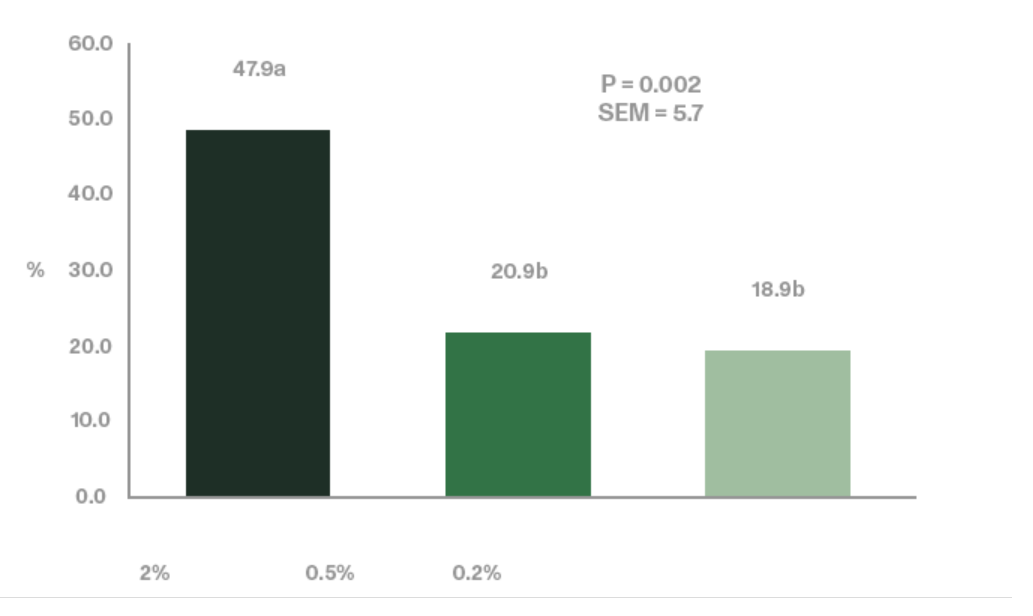
(Figure 6: dietary levels indicated in the figure have been estimated based on the content of oligosaccharides in the tested SBMs and the content of SBM in the different starter diets). © Perryman et al. (2013)
Beta-conglycinin; the overlooked danger
Beta-conglycinin is a storage glycoprotein that contains 65-80% of the protein content in Soybean meal (Murphy 2008). The carbohydrate moiety plays a large part in its immunogenicity (Amigo-Benevent et al. 2009). Beta-conglycinin is comprised of three subunits (α, α’ and β) all of which display immune-reactivity (Ogawa et al, 1995; Krishan et al. 2009; Zheng et al. 2014). Guo et al. (2008) indicated that the oral administration of beta-conglycinin α’-subunit possesses an intrinsic immuno-stimulatory capacity in rats and consumption of 5 mg/rat/day of this subunit induced an allergic response.
Beta-Conglycinin has been demonstrated to directly induce intestinal damage by inhibiting the growth of enterocytes and destroying the cytoskeleton, resulting in apoptosis (Escames et al, 2004). The effect of beta-conglycinin is dose-dependent as indicated by a linear decrease in the expression of tight-junction proteins in the gut epithelium as dietary beta-conglycinin levels increase (Zhao et al, 2014).
Several studies have corroborated that beta-conglycinin damages the integrity of the intestinal epithelium, induces inflammation, and oxidation in calves, piglets, lab animals and fish (Dreau et al., 1995; Lalles et al. 1996; Guo et al. 2008; Chen et al. 2011; Zhang et al. 2013; Peng et al. 2018). However, little attention has been given to the negative implications of soy beta-conglycinin in poultry. Nevertheless, as indicated by Kogut et al. (2018), the feed-induced sterile inflammations in poultry are purposeless energy draining responses. Thus, energy is diverted from development and growth of chickens. In young chickens, the gastrointestinal tract is immature, but develops physically, morphologically, and physiologically in the first weeks of age (De Jong et al, 2017). Reasonably, it can be deduced that any energy drain due to starter-feed-derived sterile inflammations in chickens will result in less energy available for gut development and therefore, the healthiness and competency of the intestine will be compromised for the rest of the bird's life.
The content of beta-conglycinin in solvent extracted SBM ranges from 15,000 to more than 150,000 ppm, the average being 49,430 ppm (Hamlet Protein internal data). According to these results, a 1-day-old chick that eats 13 grams of starter feed with 35% SBM will have an intake of 5-683 mg of beta-conglycinin. Likewise, a 7-day-old chick that consumes 36 grams of the starter same feed (daily estimated consumption at 7 days of life) will ingest 13-1890 mg of beta-conglycinin. These estimated intakes of beta-conglycinin in young chicks are much higher than reported by Guo et al. (2008) in rats.
Therefore, if poultry respond similar to beta-conglycinin as other species, elevated daily intake of beta-conglycinin may result in comparable immune responses that impact live performance metrics.
Conclusion
The content of soy ANF’s in broiler feed has been demonstrated to elicit a negative effect on the digestibility of the diet and the intestinal health of poultry, which both impact live performance. Frequent, effective monitoring of the ANF content in SBM and procedures to reduce levels of soy ANF’s through the minimization of conventional SBM inclusion serve as a pertinent strategies to minimize live performance losses.
| References | ||||
|---|---|---|---|---|
| 1. Gitzelmann R. and Auricchio S. | ||||
| (1965) | The handling of soy alpha-galactosidase by a normal and galactosemic child.. Pediatrics, | 36: 231–232 | ||
| 2. Marteau P. and Boutron-Ruault M.C. | ||||
| (2002) | Nutritional advantages of probiotics and prebiotics.. Br. J. Nutr. | 87: S153–S157 | ||
| 3. Coussement P.A.A. . | ||||
| (1999) | Inulin and oligofructose: safe intakes and legal status.. J. Nutr. | 129: 1412–1417 | ||
| 4. David L.T. and Peter M.C. | ||||
| (2001) | Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides.. Physiol. Rev. | 81: 1031–1064 | ||
| 5. Jiang H.Q. | ||||
| (2006) | Effect of stachyose supplementation on growth performance, nutrient digestibility and caecal fermentation characteristics in broilers.. Br. Poult. Sci. | 47: pp. 516—522 | ||
| 6. Garcia-Rebollar P. et al | ||||
| (2016) | Influence of the origin of the beans on the chemical composition and nutritive value of commercial soybean meals.. Anim. Feed Sci. Technol. | 221: 245-261 | ||
| 7. Perryman K.R. et al | ||||
| (2013) | Growth performance and meat yields of broiler chickens fed diets containing low and ultra-low oligosaccharide soybean meals during a 6-week production period.. Poult. Sci. | 92:1292–1304. | ||
| 8. Murphy P.A. | ||||
| (2008) | Soybean Proteins. In: Soybeans. Chemistry, Production, Processing, and Utilization.. AOCS Press.Published by Elsevier Inc. | 229-267 | ||
| 9. Amigo-Benavent M. et al. | ||||
| (2009) | Carbohydrate moieties on the in vitro immunoreactivity of soy β-conglycinin.. Food Res. Int. | 42: 819-825 | ||
| 10. Ogawa T.et al. | ||||
| (1995) | Alpha-subunit of betaconglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis.. Biosci. Biotechnol. Biochem. | 59: 831-813 | ||
| 11. Krishan H.B. et al | ||||
| (2009) | All three subunits of soybean beta-conglycinin are potential food allergens.. J. Agric. Food Chem. | 57: 938-943 | ||
| 12. Zheng S. et al | ||||
| (2014) | Role of soybean β-conglycinin subunits as potential dietary allergens in piglets.. Vet. J. | 199: 434-438 | ||
| 13. Guo P. | ||||
| (2008) | Recombinant soybean protein β-conglycinin α’subunit expression and induced hypersensitivity reaction in rats.. Int. Arch. Allergy Immunol. | 145: 102-110 | ||
| 14. Escames G. et al | ||||
| (2004) | Changes in iNOS activity, oxidative stress and melatonin levels in hypertensive patients treated with lacidipine.. J. Hypertens. | 22: 629−635 | ||
| 15. Zhao Y. et al | ||||
| (2014) | β-Conglycinin reduces the tight junction occludin and ZO-1 expression in IPEC-J2.. Int. J. Mol. Sci. | 15: 1915-1926 | ||
| 16. Dreau D. et al | ||||
| (1995) | T lymphocytes are enhanced in the gut of piglets fed heat-treated soybean proteins.. Vet. Immunol. lmmunopathol, | 69-79. | ||
| 17. Lalles J.P. et al | ||||
| (1996) | Feeding heated soybean flour increases the density of B and T lymphocytes in the small intestine of calves.. Vet. Immunol. lmmunopathol. | 52: 105-115. | ||
| 18. Chen F. et al | ||||
| (2011) | Soybean-derived β-conglycinin affects proteome expression in pig intestinal cells in vivo and in vitro.. J. Anim. Sci. | 89:743–753 | ||
| 19. Zhang J-X. et al | ||||
| (2013) | Soybean β-conglycinin induces inflammation and oxidation and causes dysfunction of intestinal digestion and absorption in fish.. PloS ONE 8 (3): e58115. doi:10.1371/journal.pone.0058115 | |||
| 20. Peng C. et al | ||||
| (2018) | Soybean Glycinin- and β‑Conglycinin-induced intestinal damage in piglets via the p38/JNK/NF-κB signaling pathway.. J. Agric. Food Chem. | 66: 9534−9541 | ||
| 21. Kogut M.H. et al. | ||||
| (2018) | Inflammatory phenotypes in the intestine of poultry: not all inflammationis created equal.. Poult. Sci. | 97:2339–2346 | ||
| 22. De Jong I.C. et al | ||||
| (2017) | A 'meta-analysis' of effects of post-hatch food and water deprivation on development, performance and welfare of chickens.. PLoS ONE 12(12): e0189350. https://doi.org/10.1371/journal.pone.0189350 |













