Comparison of Hydrogen Peroxide Products for Water Sanitation
Stabilised hydrogen peroxide products offer some additional residual hydrogen peroxide activity over unstabilised products but this activity lasts no more than two days, according to Tyler Clark, Brookee Dean and Susan Watkins of the University of Arkansas. They reported their latest research in the Spring 2009 issue of the University's Avian Advice.Introduction
A clean, safe water supply is essential in poultry production. Yet even producers who take every precaution to ensure that their water supply is safe may experience problems with high bacteria counts and biofilms in their water lines. Thus, it is important to understand the capabilities of water sanitation products, particularly those products capable of reducing or destroying biofilms (Hancock et al., 2007).

Hydrogen peroxide has been used as an antimicrobial agent since the early 1800s. It was used as a disinfectant in milk as early as 1904 and is presently approved by the Food and Drug Administration (FDA) for packaging and surface sterilisation in the food industry (Schurman, 2001). Hydrogen peroxide has shown to be effective against biofilms (Carpentier and Cefr, 1993).
Hydrogen peroxide (H2O2) is a weak acid that works as an oxidiser similar to chlorine. The key by-products formed when hydrogen peroxide is used are water and oxygen, which makes it a good choice for treating water with high levels of organic matter such as ponds or rivers. The hydrogen peroxide found in drugstores or pharmacies is only a three per cent concentration, while the products commonly used for water disinfection range from 16 to 34 per cent with 50 per cent H2O2 products available for use in removing biofilms from water systems between flocks. Hydrogen peroxide can also be used to oxidise iron, manganese and sulphur, which can then be removed with filtration.
The US Environmental Protection Agency (EPA) guidelines recommend 25 to 50 ppm of residual H2O2 in drinking water. However, water disinfection products use different stabilising systems, which brings us to the questions the authors are attempting to address here:
- How much of the different H2O2 concentrates is required to make a 25 to 50 ppm residual in water, and
- How long do different sources of H2O2 remain effective once they are blended into a stock solution and added to water?
Materials and Methods
The following four products were tested: hydrogen peroxide (35 per cent), HydroLine Cleaner® (34 per cent stabilised), Proxy-Clean® (50 per cent stabilised), and Oxy Blast Plus® (34 per cent stabilised). It is important to note that the HydroLine Cleaner®, Proxy-Clean® and Oxy Blast® all contain additional proprietary ingredients used for stabilisation and enhancing effectiveness. Oxy Blast® also has NSF International approval as a drinking water additive.
Each product was mixed with tap water to make four separate stock solutions of: 1, 2, 4 and 6 ounce/gallon (oz/gal) for each product. The tap water was tested for residual chlorine before mixing and measured 0 ppm. Next, one millilitre (ml) of each stock solution was added to 128 ml of tap water to create a 1:128 solution. This simulated the ounce of each stock solution that would be added to a gallon of water (128 ounces) by a medicator injecting at a 1:128 rate. After creating each of the final solutions, the parts per million (ppm) of hydrogen peroxide was tested using Oxy Blast Peroxide Test Strips which measures H2O2 residual from 0 to 100 ppm. Each solution was covered and then tested again on days 1, 2, 3, 4 and 5 post-preparation.
Results
The data in Table 1 indicate that under the conditions of this trial none of the products tested provided 25 to 50 ppm at the 1 oz/ gal stock solution level. At 2 oz/gal stock solution, hydrogen peroxide and Proxy-Clean produced 25ppm H2O2 solution, while a 4 oz/gal stock solution of HydroLine was required to produce the same concentration. A 2 oz/gal stock solution of Oxy Blast produced 50 ppm concentration of H2O2.
Assuming the products tested contained the listed percentages of hydrogen peroxide and no activity was lost in the dilution process, initial H2O2 activity for the 2 oz/gal stock solution concentration should have been 42.7, 41.5, 61.0 and 41.5 ppm for hydrogen peroxide, Hydroline, Proxy-Clean and Oxy Blast, respectively . However, the data in Table 1 suggest that in 41.5, 75.9 and 59 per cent of the H2O2 activity was lost in the initial dilution of hydrogen peroxide, HydroLine and Proxy-Clean, respectively. These data suggest that, while effective, the activity of hydrogen peroxide can be quickly lost. Therefore, it is imperative that label directions be followed when using such products.
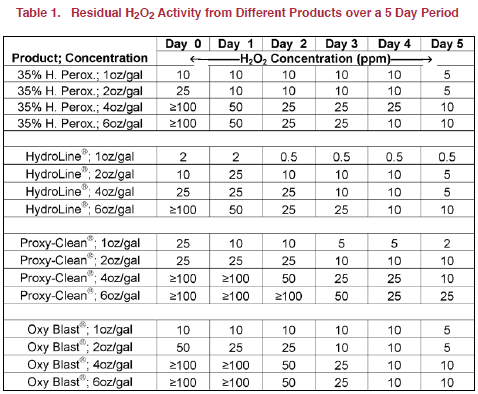
By day one or 24 hours post mix of solutions, the hydrogen peroxide at 2 oz/gal had decreased a residual H2O2 activity of 10ppm and held this concentration till day 5 when it was decreased to 5 ppm. The hydrogen peroxide at 4 oz/gal dropped to 50 ppm by day 2 and then to 25 ppm by day 3 and dropping further by day 5 to 10 ppm. HydroLine at 4 oz/gal gave a 25 ppm residual reading till Day 3 when it dropped to 10 ppm and then finished day 5 with a 5 ppm reading. The Proxy-Clean 2 oz/gal gave a 25 ppm reading till day 2 and then on day 3 it had dropped to 10 ppm for the rest of the measurement time period. The Oxy Blast 2 oz/gal mixture dropped to 25 ppm by day 1 and this held till day 3 when the residual dropped to 10 ppm. These results suggest that hydrogen peroxide, Proxy-Clean and Oxy Blast at a 2 oz/gal stock solution concentration should be adequate for providing a 25-50 ppm residual for at least 24 hours.
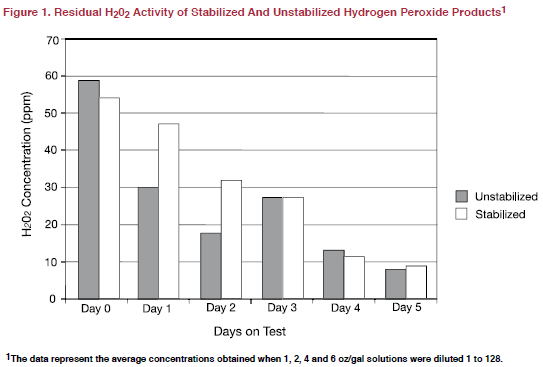
The data shown in Figure 1 compare the average residual H2O2 activity for stabilised and unstabilised hydrogen peroxide products over all concentrations tested in this trial. While both product types began and were about the same concentration on days 3, 4 and 5 of the test, stabilised products maintained higher concentrations than unstabilised products on days 1 and 2. These data suggest that stabilised hydrogen peroxide products offer some additional residual H2O2 activity when compared to unstabilised products but the additional residual activity is transient, lasting no more than one or perhaps two days.
Summary
Mixing hydrogen peroxide products to obtain a solution with a 25 to 50 ppm residual H2O2 in the drinking water required a stock solution of at least 2 oz/gal with most products. However, since hydrogen peroxide products can rapidly lose potency, it is recommended that fresh stock solutions be made every 2 to 3 days. Although stabilised hydrogen peroxide products offer some additional residual H2O2 activity over unstabilised products, this activity lasts no more than two days.
Finally, it is important to note that not all the products are labelled as drinking water additives so please take this into consideration when choosing water sanitizer products and follow label direction.
References
Carpentier, B. and O. Cefr, 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Applied Bacteriol. 75:499-511.
Hancock, A., J. Hughes and S. Watkins, 2007. In search of the ideal water line cleaner. Avian Advice 9(1):1-4.
Schurman, J.J. 2001. Antibacterial activity of hydrogen peroxide against Escherichia coli O157:H7 and Salmonella spp in fruit juices, both alone and in combination with organic acids. Thesis submitted to Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science.