Incubation Can Affect Broiler Leg Strength
Bone characteristics, serum calcium levels, early growth rate and later leg weakness could be affected by commonly used incubation programmes, according to P.J. Groves and W.I. Muir of the Faculty of Veterinary Science at the University of Sydney in their paper presented at the 2011 Australian Poultry Science Symposium.
Summary
Leg weakness in broiler chickens remains one of the major animal welfare concerns for the poultry industry worldwide, according to P.J. Groves and W.I. Muir. Recent research has indicated possible effects of incubation conditions on the skeletal integrity of the growing birds. A serendipitous finding of a field occurrence of leg weakness allowed them to target some incubation condition variations which may have been associated with this.
While an attempt to reproduce the same condition experimentally (higher temperature (0.5°C) and lower humidity (three to four per cent relative humidity) was not entirely successful, the researchers were able to demonstrate repeatable effects on bone characteristics and leg strength in broiler chickens hatched from eggs incubated under higher (0.5°C) temperature conditions. These conditions fell within the range normally acceptable for commercial broiler egg incubation.
Introduction
The aetiology of the various forms of leg weakness and lameness in the modern broiler chicken are complex, including factors relating to genetics, nutrition, infection, management and environment. The consequences for the individual bird affected and also for a considerable proportion of some flocks are serious. Bradshaw et al., 2002 stress that the welfare implications of broiler leg weakness include pain, frustration (inability to walk), reduced ability to eat and drink and consequent risk of dehydration or starvation. Birds which have difficulty in moving are also more at risk of excessive disturbance by other birds (Buijs et al., 2010) which can disrupt their sleep/rest patterns. Immobile birds are also more prone to skin damage from scratches which may result in cellulitis and death.
The underlying genetic basis associated with leg weakness is under investigation (as evidenced by Butterworth et al., 2003). Major broiler breeding companies are attempting to address many of the leg weakness issues but this requires years of genetic selection, the results of which may not be seen in the commercial broiler for many years (Elfick, 2010; Hardiman, 2010).
In the meantime, broiler producers can ameliorate the prevalence and severity of leg problems by attention to nutritional, managerial and environmental risk factors. A new area which is emerging as another possible contributor to the incidence of leg weakness problems is variation in egg incubation conditions. Research into fine-tuning incubation may provide additional management opportunities to further suppress the incidence of leg weakness and lameness.
A short review of leg weakness in broiler chickens
Lameness and leg weakness are considered a serious welfare problem. A plethora of lameness conditions in chickens exist. Bradshaw et al. (2002) summarised these into:
- infectious causes (bacterial chondronecrosis with osteomyelitis (so-called femoral head necrosis), tenosynovitis, and infectious stunting syndrome)
- developmental issues (varus-valgus deformity, tibial dyschondroplasia, rickets, chondrodystrophies and spondylolisthesis), and
- degenerative problems (osteochondrosis, epiphyseolysis, degenerative joint disease, ruptured gastrocnemius tendon and contact dermatitis).
Apart from the obvious clinical entities listed above, difficulty with locomotion is observed in birds which lack visible deformities and it has become conventional to assess the locomotory ability of birds and flocks using a standardised 'gait scoring' technique as described by Kestin et al. (1992).
A wide ranging study using gait scoring as its basis in the UK suggested that 27.6 per cent of broilers had poor locomotory ability and 3.3 per cent were unable to walk at all (Knowles et al., 2008). Many studies have not gone any deeper and the underlying pathology is often not identified. Bradshaw et al. (2002) suggested that bacterial chondronecrosis, contact dermatitis (pododermatitis) and varus-valgus deformity were the most common conditions involved. In most broiler flocks approaching slaughter age, many or all of the described conditions will be present at varying prevalence.
A detailed description of each of these conditions is beyond the scope of this paper but risk factors believed to be involved with the occurrence of the more commonly seen conditions will be summarised.
Rickets describes a condition of inadequate bone mineralisation classically induced by inadequate nutritional levels of calcium, phosphorus or vitamin D3. While broiler nutrition today is well catered for in the provision of a balance of nutrients, the occurrence of conditions which appear rickets-like (soft bendable bones and beaks) is seen commonly in young chicks. Clinical rickets can be seen following occurrences of infectious stunting syndrome (ISS) in flocks, relating to poor absorption of nutrients associated with indigestion induced by the group of viruses. ISS immunity is poorly understood and although the flock condition occurs sporadically, the viruses involved should be expected to be widely present in the broiler environment. One wonders about the possibility that subclinical ISS in many flocks may play a part in subsequent skeletal problems on a wide scale.
Tibial dyschondroplasia (TD) is a disruption of normal ossification as bones grow. An interference with adequate blood supply in the metaphysis of the tibiotarsus results in insufficient nutrients reaching the growth plate and a cartilage plug forms which fails to be ossified. Bones are subsequently weak, may bend and cause considerable pain in weight bearing. Genetics, incorrect electrolyte balance in feed and mycotoxins have been implicated in TD development. It is a commonly seen entity in broilers and is often correlated with an imbalance of the calcium:phosphorus ratio in the feed, compounded by the difficulty in predicting real available phosphorus levels from available ingredients with and without phytase supplementation.
It is quite feasible that the presence of earlier degenerative conditions, especially rickets-like conditions, may predispose birds to the appearance of other conditions later in the flock's life. In the field, rotated tibia is becoming one of the major leg deformities seen. The aetiology of this condition is not known but early rickets may be a predisposing factor (Crespo & Shivaprasad, 2008). Thorp (2008) also implicated the earlier occurrence of rickets or dyschondroplasia with varus-valgus deformity.
Many of the leg weakness conditions can be modified by management and environmental conditions. Field and laboratory studies, however, are sometimes contradictory in the effects observed. Stocking density has often been implicated with an increased incidence of leg problems (Knowles et al., 2002; Bradshaw et al., 2002; Petek et al., 2010) while other studies have shown leg problems to peak at intermediate levels rather than higher stocking densities (Buijs et al., 2009; Hepworth et al., 2010), or to not be related to stocking density at all (Dawkins et al., 2004).
Lengthy photoperiod has also been incriminated with a higher incidence of leg weakness (Brickett et al., 2007; Bradshaw et al., 2002; Knowles et al., 2008; Petek et al., 2010) as has lack of exercise (Cooper and Wrathall, 2010; Sherlock et al., 2010) which has a relationship to scotoperiod (the length of the dark period). Many relate the primary risk factors to growth rate (Knowles et al., 2008, Bradshaw et al., 2002; Sherlock et al., 2010). Maintenance of dry litter conditions also can have major effects on pododermatitis (Sherlock et al., 2010). Modification of these factors can lead to better outcomes for broiler leg health.
More recent work, including that reported herein, has demonstrated associations of variations in incubation conditions and subsequent leg strength and this will be summarised below.
* "Fine-tuning incubation may provide additional management opportunities to further suppress the incidence of leg weakness and lameness" |
Links to incubation condition
Recent published reviews and research have implicated defects in incubation as possible contributors to some bone irregularities in broiler chickens or turkeys. Spraddle legs in broilers have been associated with high humidity during incubation (Crespo & Shivaprasad, 2008), and Genin et al. (2008) implicated cyclic overheating during the first eight days of incubation in the later incidence of tibial dyschondroplasia via an effect on growth plate hypoxia.
Oviedo-Rondon et al. (2008) showed that pre-heating conditions of eggs prior to incubation could affect bone characteristics of chicks at hatch and the incidence of twisted legs as late as 40 days of age. These authors also described effects on bone development and characteristics following early cool and/or late high temperature profiles and low oxygen tensions used during parts of the incubation process. Soft tissue effects have also been seen. In further experiments, Oviedo-Rondon et al. (2010) demonstrated an effect of an early low and later high incubation temperature profile in producing thinner gastrocnemius tendon fibres and differing collagen banding patterns during subsequent growth. The temperatures used in these studies though were outside the normal realms of incubation practice (36°C and 39°C).
The local field observations have suggested a possible effect of incubation differences on subsequent leg strength and these will be discussed below.
Commercial hatcheries run differing incubation profiles depending on their machine type and whether these run as single or multi-stage incubation. Multi-stage incubators target a single temperature and humidity profile usually between 36.9 and 37.2°C and relative humidity between 51 and 65 per cent.
Single-stage commercial incubation uses a decreasing temperature profile starting at 38°C and decreasing to 37.2°C by 18 days with relative humidities varying between 50 to 58 per cent and sometimes as wide as 30 to 65 per cent.
The experimental profile used in these studies was within these commercially used bounds and basically employed a higher temperature (0.5°C) over later incubation, a lower relative humidity (three per cent) between days 7 to 18, and a pulse reduction in temperature at day 6 of 1°C.
The objectives of this research were to determine whether the variation in incubation conditions described generated a higher incidence of early bone weakness in newly hatched chicks and to then evaluate if later skeletal deformities or leg weakness could be associated with the incubation profile.
Materials and Methods
Experiment 1 used 2,000 and in experiment 2, 560 fertile eggs from breeders of a fast feathering dam line. In each experiment, the eggs were randomised between two incubators. The incubators were set to operate differently up to 18 days of incubation as shown in Figures 1 to 4. The major intended differences were an approximate drop in temperature of about 1°C for one day at six days of incubation, a higher continuous temperature from seven to 18 days of incubation (0.5°C) and a lower relative humidity (three per cent) throughout. These settings were based on an observed field situation where chicks with poor bone quality at hatch were produced, compared to an 'ideal' incubation profile as the control (Jan Meldrum, personal communication). From 18 days of incubation, all eggs were transferred into a common incubator set at 36.9°C and reduced by 0.3°C per day until day 21. Temperature and humidity data loggers (AZ 8829) recording conditions at hourly intervals were placed in each machine amongst the eggs.
At hatch, 44 randomly selected chicks from each incubator group were blood sampled for serum calcium and phosphorus levels and then humanely euthanised and both femurs were collected for bone ash analysis. Remaining chicks were placed in floor pens (240 birds per large pen in experiment 1 and 45 birds per smaller pen in experiment 2) and grown on commercial broiler starter and finisher rations (0-21 days and 22-42 days respectively) supplied by Millmaster Feeds, Enfield, New South Wales.
At two weeks of age, 40 or 44 birds were randomly selected from each group, blood sampled for serum calcium and phosphorus levels and humanely euthanised. The proximal ends of their left tibiae were longitudinally sectioned and the epiphyseal growth plate measured at the midpoint of the bone with a digital calliper. The left femurs were collected for bone ash analysis.
At day 28 in experiment 1, 44 birds were randomly selected and euthanised. The proximal end of their left tibiae were sectioned longitudinally and scored for the presence of tibial dyschondroplasia (TD) lesions (on a scale of 0 to 4, where 0 = no lesion and 4 = large lesion spanning the entire growth plate).
At six weeks of age, 40 or 50 randomly selected chickens from each group were submitted to a Latency-to-Lie (LTL) test (first described by Weeks et al., 2002 and modified by Berg and Sanotra, 2003) for a maximum of five minutes. In experiment 1, a random sample of 30 birds per pen was weighed at 14, 21, 28, 35 and 42 days. In Experiment 2, all birds were weighed on a pen basis at 7, 21, 28, 35 and 42 days.
Where data were normally distributed, comparisons were made using Analysis of Variance (ANOVA) where independent variables included incubator and sex and were compared across both experiments. Where data were not normally distributed, the Mann-Whitney U test was used to separate main effect means. LTL tests were compared using Kaplan-Meier Survival Analysis.
Results
The incubation temperature and relative humidity profiles recorded by the data loggers in each machine (actual) compared to the intended profiles are shown respectively in Figures 1 to 4. In experiment 1, the control incubator ran slightly cooler than intended and its humidity was not well controlled. In experiment 2, intended temperatures were much better matched but humidity was lower than intended and similar in both incubators.
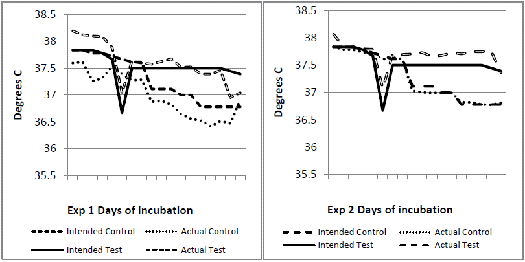
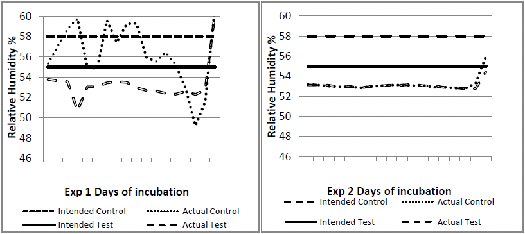
At hatch, chicks from the test incubator profile delivered consistently and significantly lower femoral bone ash percentage and higher serum calcium levels than the control profile (Table 1). Moisture loss from both test treatments was significantly higher (Table 1). In experiment 1, in which the test treatment maintained a three to four per cent lower relative humidity, the serum calcium was lower than the serum phosphorus for both control and test treatment (Table 1). At two weeks of age, serum phosphorus exceeded serum calcium in all four groups but this ratio was again consistently higher for experiment 1. At two weeks, there was a significant interaction for bone ash percentage between the two experiments (Table 2), indicating a different response in this parameter under the differing incubation conditions that actually occurred in the incubators.
| Table 1. Hatch measurements | |||||
|---|---|---|---|---|---|
| Incubator group | Total hatchability (% ± SE) |
Egg weight loss to day 18 of incubation (% ± SE) |
Femoral bone ash (% ± SE) |
Serum Ca (mmol/l ± SE) |
Serum P (mmol/l ± SE) |
| Exp 1 Control Exp 2 Control |
67.92 ± 5.74 77.8 ± 4.02 |
9.45 ± 0.14 10.12 ± 0.26 |
26.9 ± 0.63 28.3 ± 0.40 |
1.97 ± 0.04 2.17 ± 0.04 |
2.31 ± 1.32 1.22 ± 0.03 |
| CONTROL MEAN | 72.86 | 27.4A ± 0.38 | 2.01B ± 0.03 | 1.93 ± 0.85 | |
| Exp 1 Test Exp 2 Test |
71.28 ± 1.11 75.1 ± 2.75 |
10.28 ± 0.14 11.17 ± 0.27 |
25.3 ± 0.63 27.5 ± 0.28 |
2.10 ± 0.04 2.28 ± 0.02 |
2.30 ± 1.25 1.35 ± 0.03 |
| TEST MEAN | 73.19 | 26.1B ± 0.43 | 2.16A ± 0.03 | 1.97 ± 0.82 | |
| P= | 0.04 | 0.002 | 0.87 | ||
| Table 2. Measurements at two weeks of age | |||||
|---|---|---|---|---|---|
| Incubator group | Bird weight (g ± SE) |
Tibial growth plate width (mm ± SE) |
Femoral bone ash (% ± SE) |
Serum Ca (mmol/l ± SE) |
Serum P (mmol/l ± SE) |
| Exp 1 Control Exp 2 Control |
401 ± 5.55 393 ± 5.66 |
2.05 ± 0.05 2.16 ± 0.08 |
44.1 ± 0.24 44.7 ± 0.21 |
1.69 ± 0.05 2.06 ± 0.04 |
2.03 ± 0.06 2.30 ± 0.05 |
| CONTROL MEAN | 397B ± 3.80 | 2.10B ± 0.06 | 44.4 ± 0.16 | 1.88 ± 0.04 | 2.17 ± 0.04 |
| Exp 1 Test Exp 2 Test |
408 ± 5.98 413 ± 5.66 |
2.38 ± 0.07 2.36 ± 0.08 |
43.0 ± 0.30 45.7 ± 0.24 |
1.75 ± 0.07 2.12 ± 0.05 |
2.01 ± 0.07 2.37 ± 0.04 |
| TEST MEAN | 410A ± 4.00 | 2.37A ± 0.06 | 44.3 ± 0.24 | 1.93 ± 0.05 | 2.19 ± 0.04 |
| P= | 0.02 | 0.001 | 0.68 | 0.41 | 0.75 |
| A,B means with different superscripts differ (P<0.05) | |||||
Growth rate over the first two weeks was significantly greater in the test profile incubated chicks in both experiments but weights after this age were similar (Table 3).
| Table 3. Growth rates | ||||||
|---|---|---|---|---|---|---|
| Incubator group | Mean body weights (g ± SE) | |||||
| Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
| Exp 1 Control | 128 ± 1.91 | 381 ± 3.97 | 818 ± 10.1 | 1398 ± 14.4 | 2084 ± 9.4 | 2665 ± 38.4 |
| Exp 2 Control | 162 ± 1.09 | 393 ± 3.24 | 834 ± 9.7 | 1408 ± 20.3 | 2057 ± 25.8 | 2636 ± 40.9 |
| CONTROL MEAN | 145B ± 6.52 | 386B ± 3.34 | 825 ± 20.1 | 1403 ± 11.7 | 2071 ± 13.6 | 2650 ± 26.6 |
| Exp 1 Test | 138 ± 4.50 | 400 ± 10.9 | 812 ± 13.2 | 1363 ± 35.2 | 2113 ± 27.8 | 2703 ± 26.9 |
| Exp 2 Test | 172 ± 2.19 | 413 ± 13.8 | 863 ± 15.8 | 1441 ± 43.7 | 2094 ± 27.7 | 2676 ± 38.6 |
| TEST MEAN | 155A ± 7.00 | 407A ± 6.5 | 837 ± 13.7 | 1402 ± 24.2 | 2104 ± 18.5 | 2690 ± 22.3 |
| P= | <0.001 | 0.04 | 0.05 | 0.21 | 0.45 | 0.64 |
| A,B means with different superscripts differ (P<0.05) | ||||||
Survival Analysis for the Latency to Lie test results show that birds from the test incubator groups had significantly shorter LTL time (median 94 seconds compared to 136.5 seconds for the control group, P=0.0002, Gehan's Wilcoxon test) and had fewer birds that managed to remain standing for the full five minutes.
Discussion and Conclusions
Although different, the intended temperature profiles used in both incubators fell within acceptable limits for successful incubation (37.1 to 38.2°C; Hill, 2010) Relative humidity was much harder to control with the incubators used. The incubators used in this preliminary work were semi-commercial types, not machines designed to provide fine control necessary for experimental work. Although the incubators did not perform completely as intended, particularly the control machine, significant differences between the chicks from each incubator profile were observed in chick bone ash and serum calcium at hatch and at growth rate to two weeks of age, and this was relatively consistent. The overall higher incubation temperature in the test treatments appears to have increased moisture loss from fertile eggs as well as embryonic growth, with commensurate impacts on bone ash and serum calcium.
High early growth rate has been implicated as contributing to leg weakness problems for some time (Bradshaw et al., 2002; Brickett et al., 2007; Knowles et al., 2008) and the overall increased early growth seen associated with the test incubation profile here may have had its effect on LTL when the birds were older. The two experiments show that bone characteristics, serum calcium levels, early growth rate and later leg weakness could be affected by incubation programmes within the usually acceptable hatchery range.
Acknowledgements
This work was funded by the Australian Poultry Cooperative Research Centre.
The authors are indebted to the excellent technical assistance provided by Mrs Joy Gill, Mrs Melinda Hayter and Mr Todd Gill in performing the incubations, care and handling of the birds and assistance with sample collections. Mrs Gill performed the bone ash measurements. Ms Ball and Mrs Sharpe assisted with some of the sampling procedures.
References
Berg, C. and Sanotra, G.S. 2003. Animal Welfare, 12:655-659.
Bradshaw, R.H., Kirkden, R.D. and Broom, D.M. 2002. Avian and Poultry Biology Reviews, 13(2) May:45-103.
Brickett, K.E., Dahiya, J.P., Classen, H.L., Annett, C.B. and Gomis, S. 2007. Poultry Science 86:2117-2125.
Buijs, S., Keeling, L., Rettenbacher, S., Van Pouke, E. and Tuyttens, F.A.M. 2009. Poultry Science 88:1536-1543.
Buijs, S., Keeling, L.J, Vangestel, C., Baert, J., Vangetye, J., Andre, F. and Tuyttens, M. 2010. Applied Animal Behaviour Science, 124(3-4):97-103.
Butterworth, A., Reeves, N.A., Knowles, T.G. and Kestin, S.C. 2003. Animal Welfare. 12(4):661-667(7)
Cooper, M. and Wrathall, J. 2010. Animal Welfare 19(S):51-56.
Crespo, R. and Shivaprasad, H.L. 2008. In: Diseases of Poultry, 12th Ed. Saif, Y.M. Ed. Blackwell Publishing, Ames, Iowa. 1160-1162.
Dawkins, M.S. et al., 2004. Nature 427:342-344.
Elfick, D. 2010. Proceedings of the New Zealand Poultry Industry Conference 10:52-63.
Genin, O., Hasdai, A., Yalcin, S. and Pines, M. 2008. Proceedings of the World's Poultry Congress, Brisbane, Australia 258
Hardiman, J. 2010. Proceedings AVPA meeting, Christchurch, New Zealand. October 14-15.
Hepworth, 2010. Avian Pathology 39(5) Oct:405-409.
Hill, D. 2010. Proceedings AVPA Scientific meeting, Gold Coast, Queesland, Australia.
Kestin, S.C., Knowles, T.G., Tinch, A.E and Gregory, N.G. 1992. Vet Record, 131:190-194.
Knowles, T.G, Kestin, S.C., Haslam, S.M., Brown, S.N., Green, L.E., Butterworth, A., Pope, S.J., Pfeiffer, D and Nicol, C.J. 2008. PLoS ONE, 3(2):e1545. doi:10.1371/journal.pone.0001545
Oviedo-Rondon, E.O., Wineland, M.J., Christensen, V.L., Brake, J., Small, J. and Cutchin, H. 2008. Proceedings of the World's Poultry Congress, Brisbane, Australia 258.
Oviedo-Rondon, E.O., Halpar, J., Wineland, M.J., Ferket, P., Cummock, J. and Kusovschi, A. 2010. Proceedings AAAP meeting, Atlanta, August 1-4.
Petek, M., Cibik, R., Yildiz, H, Sonat, F. A., Gezen, S.S., Orman, A. and Aydin, C. 2010. Veterinarija Zootechnika (Vet Med Zoot) T. 51(73).
Sherlock, L., Demmers, T.G.M, Goodship, A.E., McCarthy, I.D. and Wathes, C.M. 2010. British Poultry Science 51(1):22-30.
Thorp, B.H. 2008. In: Poultry Diseases, 6th Ed. Elsevier. 470-482
Weeks, C.A., Knowles, T.G., Gordon, R.G., Kerr, A.E., Peyton, S.T. and Tilbrook, N.T. 2002. Vet Record, 151:762-764.
Further Reading
| - | You can view other papers presented at the Australian Poultry Science Symposium 2011 by clicking here. |
August 2011