



A Reappraisal of the Potential of Dietary Fatty Acids to Ameliorate Heat Stress
The strategic use of new oil seed varieties high in oleic acid may ameliorate the adverse effects of heat stress in poultry, according to P.J. Cronjé of Cronjé Consulting and Editing at the 2011 Australian Poultry Science Symposium.
Summary
Although the concept of manipulating dietary fat content to ameliorate the effects of heat stress in poultry, pigs and cattle seems reasonable on theoretical grounds, it has yielded mixed results in all species. However, little attention was given to the fatty acid composition of the fat sources used, presumably because the premise on which this strategy is based was that all lipid sources have lower heat increments than the dietary carbohydrates that they replace.
Nevertheless, there is evidence that dietary supplementation with long-chain fatty acids such as palmitic, linoleic and oleic acid can ameliorate the adverse effects of high temperatures in poultry (Njoku and Nwazota, 1989; Balnave, 1998; Mujahid et al., 2009).
Recent advances in the elucidation of the mechanism by which hyperthermia exerts its effects strongly suggest that up-regulation of avUCP expression using specific fatty acids may prevent the cascade of events that results in decreased production and tissue damage during heat stress.
Furthermore, recent studies on the pathophysiology of heat stress strongly suggests that the strategic use of new oil seed varieties high in oleic acid may ameliorate the adverse effects of heat stress in poultry.
Introduction
Dietary fat is metabolised with greater efficiency than dietary carbohydrate or protein. Thus, less heat is generated during the metabolism of dietary fat than during the metabolism of dietary carbohydrate or protein. A logical application of this concept to the nutrition of livestock is replacement of a portion of the diet with fat to decrease dietary heat increment under heat stress conditions. Despite many studies on the inclusion of various sources of fat in the diets of dairy cows exposed to hot environmental conditions, several reviews on this topic concluded that the literature on the benefits of fat supplementation during heat stress is inconclusive (Beede and Collier, 1986; Huber et al., 1994; West, 1999). Similarly, the NRC (1981) reviewed the literature on the addition of fat to poultry diets fed during heat stress and concluded that this practice has not been consistently successful.
More recently, Balnave (2004) noted that as the type of fat affects nutrient partitioning to adipose tissue in broilers, interactions between environmental temperature and fat source may be worth exploring. Explication of the disparities between studies on the use of high fat diets during heat stress is difficult because sources of fat differ and their fatty acid composition is often not defined. Although no systematic study of the effects of different dietary fatty acids on animal responses to heat stress has been conducted to date, a remarkable series of studies conducted by Toyomizu’s group at Tohoku University in Japan on the pathophysiology of heat stress in poultry strongly suggests that the adverse effects of heat stress could be alleviated by strategic supplementation with specific fatty acids (Mujahid et al., 2005, 2006, 2007a, 2007b, 2007c, 2009).
The aim of this review is to discuss recent advances in our understanding of pathology of heat stress in poultry and to determine whether specific dietary fatty acids could play a role in ameliorating heat stress in poultry.
Fatty Acids are Involved in Pathology of Heat Stress
Although the reduction in feed intake that accompanies heat stress undoubtedly contributes to a decrease in production under hot conditions, it has been demonstrated that it is only responsible for half the reduction in growth rate in broilers (Geraert et al., 1996a).
In contrast to the expected effect of decreased feed intake on adipose tissue, heat stress increases the mass of certain fat deposits by 33 to 64 per cent (Geraert, 1998). Furthermore, the fatty acid composition of adipose tissue is altered by heat stress (Geraert, 1998). Cells of the heart, kidney and liver of heat-stressed broilers exhibit an abnormally high accumulation of lipid droplets in the cytoplasm and massive fatty degeneration (Aengwanich and Simaraks, 2004).
A similar pathology was described by Butler (1976) for fatty liver haemorrhagic syndrome, a condition that occurs when layers are exposed to hot weather: the liver is putty coloured and grossly enlarged because of excessive fat infiltration, which accumulates as globules within the cell to the extent that the nucleus is displaced and some cells are ruptured.
Heat stress increases levels of plasma fatty acids (Mujahid et al., 2007b), triglycerides (Sahin et al., 2006), cholesterol (Sahin et al., 2006) and enzymes involved in the transport and oxidation of fatty acids (Mujahid et al., 2007b). The respiratory quotient of heat-stressed birds is decreased (Mckee et al., 1997) indicating that hyperthermia promotes oxidation of fatty acids. It is thought that fatty acid oxidation is increased to meet the energy requirements of birds exposed to heat stress (Mckee et al., 1997). However, the pathology of heat stress is indicative of an imbalance between mobilisation of fatty acids and the ability to oxidise them. Excessive fatty acid oxidation and accumulation of fatty acids in mitochondria is conducive to oxidative stress, a condition that causes significant tissue damage.
Hyperthermia Causes Oxidative Stress
Oxidative stress is characterised by excessive production of reactive oxygen species (ROS) such as superoxide. ROS remove electrons from fatty acids, mainly polyunsaturated fatty acids, creating fatty acid radicals that in turn attack other fatty acids. This process is called lipid peroxidation. If left unchecked, such chain-reactions damage cell membranes, which consist mainly of lipids, resulting in impaired control of cellular ion homeostasis and eventually, cell death. ROS also damage proteins and DNA.
Prolonged heat-induced oxidative stress initiates a cascade of events involving systemic elevation of levels of inflammatory cytokines, widely disseminated intravascular blood coagulation and ultimately, multiple organ failure and death (for review, see Cronje, 2005). In broilers, exposure to five hours of heat stress per day (33°C and 60 to 70 per cent relative humidity) for 21 days resulted in symptoms consistent with excessive oxidative stress: congestion, oedema and haemorrhage of the lungs, oedema and haemorrhage of the kidneys and necrosis of the liver (Aengwanich et al., 2003; Aengwanich and Simaraks, 2004). Heat stress also causes haemorrhages in muscle tissue (Sandercock et al., 2001) and damage to the intestinal mucosa (Quinteiro-Filho et al., 2010) in poultry.
There is ample evidence showing that heat stress results in oxidative stress in poultry (Altan et al., 2003; Sahin et al., 2006; Feng et al., 2008) and that it causes extensive damage to lipids, proteins (Mujahid et al., 2007a) and muscle membranes (Sandercock et al., 2001; Petracci et al. 2009). Oxidative stress arises when the body’s natural antioxidant defences are unable to cope with ROS generated during oxidative phosphorylation in the mitochondria. Several studies have shown that vitamins and minerals involved in antioxidant defence are depleted by heat stress (Sahin et al., 2003; Mahmoud and Edens, 2005). That supplementation of heat-stressed birds with antioxidants such as vitamin C (Mckee et al., 1997; Sahin et al., 2003; Mahmoud et al., 2004), vitamin E (Bollenger-Lee et al., 1998) and lycopene (Sahin et al., 2006) has been shown to ameliorate heat-induced oxidative stress is a strong indication that heat stress induces over-production of ROS.
In 2005, Mujahid et al. demonstrated for the first time that heat stress induces the production of superoxide in the skeletal muscle mitochondria of broilers and showed that oxidative stress inhibits growth independently of feed intake during heat stress. Therefore, nutritional strategies against heat stress such as increased dietary energy density or protein content only address half the problem (decreased feed intake) and strategies such as supplementation with antioxidants only address the symptoms of the other half of the problem (oxidative stress). A strategy that targets the cause of oxidative stress is lacking. However, the cause of oxidative stress in poultry remained a matter of conjecture until the discovery of avian uncoupling protein by Raimbault et al. in 2001.
Mitochondrial Uncoupling Proteins Decrease Oxidative Stress
Hydrolysis of ATP to ADP releases energy, which is used to drive metabolic reactions. An active cell can hydrolyse more than two million ATP molecules per second, but the energy stored in the form of ATP in the human body is equivalent to the energy stored in an AA battery, and therefore only sufficient to satisfy the body’s energy needs for a few seconds. This necessitates rapid regeneration of ATP from ADP using energy from ingested nutrients or endogenous reserves. Thus, although the human body contains only 250 g of ATP, it turns over its own weight in ATP each day. The task of ATP turnover is accomplished by mitochondria, of which there are 100 to 1,000 per cell. Most ATP is produced in mitochondria by oxidative phosphorylation.
A schematic illustration of mitochondrial oxidative phosphorylation is presented in Figure 1. The mitochondrion contains an inner and an outer membrane, which are separated by an inter-membrane space. In the matrix of the mitochondrion, oxidation of glucose and fat yields the ‘hydrogen carriers’ NADH+H+ and FADH2. During oxidative phosphorylation, electrons are removed from NADH+H+ and FADH2 and are transported through the respiratory chain until they are donated to molecular oxygen, which is then reduced to water. The transport of electrons drives proton pumps that transfer hydrogen ions from the matrix to the inter-membrane space, creating an electrochemical potential difference across the inner membrane. Protons may re-enter the mitochondrial matrix through the ATP synthase proton channel, which uses this proton-motive force to generate ATP from ADP.
Proton re-entry via ATP synthase is normally regulated by the availability of ADP but protons may also re-enter through uncoupling proteins (UCPs), which act as a type of 'pressure-relief valve' to prevent excessive accumulation of protons in the inter-membrane space. During the reduction of molecular oxygen to water, leakage of electrons from the respiratory chain results in the formation of superoxide radicals, which can be converted into other ROS. These ROS attack the phospholipids and polyunsaturated fatty acids (PUFA) of the inner membrane. Thus, activation of UCPs, which enables protons to leak back into the matrix, reduces ROS production (Azzu and Brand, 2009). As mitochondria account for more than 80 per cent of cellular oxygen consumption, they are the main site of ROS production (Manoli et al., 2007). When the level of ROS exceeds the capacity of cellular antioxidants to remove them, the cell experiences oxidative stress. If left unchecked, DNA and enzymes are damaged and the respiratory chain malfunctions.
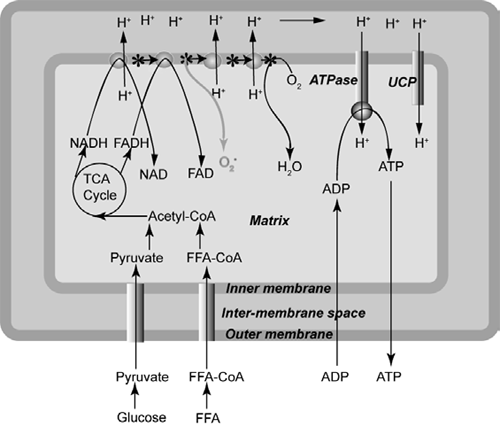
Glucose and free fatty acids (FFA) enter the tricarboxylic acid (TCA) cycle as acetyl-CoA, producing NADH and FADH, which donate electrons to the electron transport chain. Movement of electrons down this chain provides energy to transport protons (H+) from the matrix to the inter-membrane space, creating a proton electrochemical gradient. Re-entry of protons to the matrix via ATP synthase drives the conversion of ADP to ATP. Electrons that reach the end of the electron transfer chain are accepted by molecular oxygen (O2) in the formation of H2O. However, some electrons leak from the chain and form superoxide (O2-).
In addition to its role in decreasing ROS by increasing proton leak, it has been proposed that UCP3 exports fatty acids from the mitochondrial matrix when fatty acid supply exceeds fat oxidation capacity (Hoeks et al., 2003). As fatty acid anions in the mitochondrial matrix are prone to peroxidation, prevention of their accumulation could reduce ROS production. Although there is debate about the relative importance of the various mechanisms by which UCP3 exerts its effects (Azzu and Brand, 2009), there appears to be general consensus that UCP3 plays a key role in decreasing ROS production and protecting against cellular damage. Therefore, heat-induced down-regulation of UCP activity could provide an explanation for the oxidative stress observed in birds subjected to heat stress.
Avian Coupling Protein is Downregulated by Heat Stress
Only one type of UCP has been detected in birds, whereas five iso-forms are present in mammals. Avian uncoupling protein (avUCP) was first cloned in 2001 by Raimbault et al. from the skeletal muscle of chickens. The amino acid sequence of avUCP is 70 per cent identical with those of mammalian UCP2 and UCP3, but its tissue distribution is restricted mainly to skeletal muscle, which is similar to the distribution of UCP3.
Mujahid et al. (2006, 2007b,c) showed that heat stress decreases the level of avUCP by up to 50 per cent and proposed that the associated inability to regulate proton motive force caused oxidative stress. They also observed that plasma fatty acid levels increased three-fold and that levels of enzymes involved in the transport and oxidation of fatty acids and those involved in the Kreb's cycle were elevated during the early stages of heat stress (Mujahid et al., 2007b). The same group recently showed that heat stress enhances substrate oxidation via the electron transport chain, resulting in an increase in mitochondrial membrane potential and ROS production (Kikusato et al., 2010). Mujahid et al. (2007b) concluded that a sudden surge in mitochondrial substrate oxidation combined with down-regulation of avUCP may be responsible for the increase in superoxide production during heat stress. This hypothesis is supported by further evidence of down-regulation of avUCP by heat stress in chickens (Taouis et al., 2002) and a very strong linear correlation (R2 = 0.92) between ROS production and avUCP-dependant mitochondrial proton leak (Rey et al., 2010). However, the mechanism by which heat stress down-regulates avUCP is as yet unclear.
The recent identification of a binding site for thyroid hormone in the promoter sequence of the avUCP gene by Joubert et al. (2010) indicates that thyroid hormone may play a role in downregulating avUCP expression during heat stress.
Changes in Thyroid Hormone Level May Downregulate Avian Uncoupling Protein Expression During Heat Stress
During heat stress, circulating levels of thyroid hormone are decreased (Geraert et al., 1996b; Tao et al., 2006; Lin et al., 2008), presumably because thyroid hormone increases metabolic rate, and thus metabolic heat production. Although it has been known for many years that thyroid hormone stimulates metabolic rate and decreases metabolic efficiency, the mechanism by which thyroid hormone affects energy homeostasis is poorly understood. In 2001, De Lange et al. provided the first in vivo evidence that thyroid hormone increases muscle UCP3 expression.
More recently, Rey et al. (2010) showed that skeletal muscle avUCP abundance in ducklings was up-regulated by administration of thyroid hormone and decreased by pharmacological blockade of thyroid hormone synthesis. Furthermore, the production of ROS per unit of oxygen consumed by muscle mitochondria was elevated in the hypothyroid state and was attenuated by thyroid hormone administration. In rats, thyroxine level is linearly correlated with muscle UCP3 expression (Sprague et al., 2007).
Therefore, a heat-induced decrease in thyroid hormone level may down-regulate avUCP expression, resulting in oxidative stress and ROS-mediated tissue damage in birds exposed to heat stress. There is evidence that polyunsaturated fatty acids up-regulate UCP expression and that they compete with thyroid hormone for the retinoid receptor X, which is required for binding to some of their target genes (Clarke et al., 1999). Thus, it is possible that dietary mono- and poly-unsaturated fatty acids could be exploited to increase avUCP expression and ameliorate heat-induced tissue damage in poultry.
Fatty Acids Upregulate Uncoupling Protein Expression
Muscle UCP3 up-regulation appears to be specific for long-chain fatty acids, as Hoeks et al. (2003) observed no response in muscle UCP3 level when rats were fed a high-fat diet consisting of medium-chain fatty acids, but observed substantial increases when a diet containing long-chain fatty acids was fed.
Thompson et al. (2004) reviewed in vitro studies in which specific fatty acids had been added to cultured cell models or primary isolated cells. None of the cell lines showed a response in UCP3 expression to saturated fatty acids. In muscle cells, the mono-unsaturated fatty acid, oleic acid (18:1 n–9) and the polyunsaturated fatty acids, linoleic acid (18:2 n–6) and linolenic acid (18:3 n–3) increased the expression of UCP3.
Rodriguez et al. (2002) fed rats diets containing 40 per cent of dietary energy in the form of oils rich in saturated fatty acids (palm oil or beef tallow), polyunsaturated fatty acids (sunflower oil) or mono-unsaturated fatty acid (olive oil). The level of UCP3 in muscle was 33 per cent greater in rats fed the olive oil diet than in those fed the other sources of fatty acids.
Based on this evidence, Mujahid et al. (2009) fed olive oil to broilers to determine whether it Rodriguez et al. (2002) fed rats diets containing 40 per cent of dietary energy in the form of oils rich in saturated fatty acids (palm oil or beef tallow), polyunsaturated fatty acids (sunflower oil) or mono-unsaturated fatty acid (olive oil). The level of UCP3 in muscle was 33 per cent greater in rats fed the olive oil diet than in those fed the other sources of fatty acids.
Based on this evidence, Mujahid et al. (2009) fed olive oil to broilers to determine whether it could prevent mitochondrial ROS production and oxidative damage during heat stress. In their trial, birds were fed a basal diet (a commercial broiler diet) or the basal diet plus 6.7 per cent olive oil for eight days before exposure to thermoneutral conditions or 34°C for 12 hours. The addition of olive oil to the basal diet prevented the decrease in avUCP level and the increase in lipid peroxidation observed in birds fed the control diet during heat stress. Birds fed the basal diet lost weight during heat stress, whereas those supplemented with olive oil gained weight. The feed intake of the olive oil-supplemented birds also decreased to a lesser extent than that of birds fed the basal diet during the 12 hours of heat stress period. Although the practical implications of these results are difficult to interpret because the two diets were not isoenergetic, it establishes a mechanism by which specific fatty acids could alleviate heat stress. As oleic acid, a mono-unsaturated fatty acid, constitutes 70 to 80 per cent of the fatty acids in olive oil (Tripoli et al., 2005), it is likely that the up-regulation of avUCP observed by Rodriguez et al. (2002) and Mujahid et al. (2009) was mediated by oleic acid.
The specificity of UCPs for certain types of fatty acid may explain why the practice of feeding high-fat diets to poultry exposed to heat stress has been successful in some instances and has failed in others. Furthermore, Hoeks et al. (2003) noted that rats fed a high-fat diet containing medium-chain fatty acids (C8:0 and C10:0; caprylic and capric acid, respectively) gained less weight than rats consuming an equal amount of net energy from a high-fat diet containing long-chain fatty acids (C16:0, palmitic acid), indicating that medium-chain fatty acids have a thermogenic effect.
Therefore, it is possible that supplementation of poultry diets with certain types of fatty acids could exacerbate heat stress. The long-chain fatty acid diet – but not the medium-chain fatty acid diet – increased UCP3 level in muscle. It is noteworthy that the aforementioned thermogenic effect of medium-chain fatty acids occurred in the absence of up-regulation of UCP3, supporting the contention of Hoeks et al. (2003) that UCP3 does not increase heat production but protects mitochondria against fatty-acid-induced mitochondrial damage. Baillie et al. (1999) observed that fish oil, which contains long-chain omega-3 C20 and C22 polyunsaturated fatty acids, resulted in less fat deposition in rats fed equicaloric amounts of a diet containing corn oil (rich in C18:2 [n-6]; omega-6 linoleic acid), showing that long-chain fatty acids can have thermogenic effects. In this instance, the thermogenic diet (fish oil) also increased muscle UCP3 level to a greater extent than the corn oil diet. The differential effects of various fatty acids on thermogenesis and UCP expression may be mediated by their affinity for peroxisome proliferator-activated receptors (PPARs).
The PPARs were originally identified in frogs as receptors that induce the proliferation of peroxisomes, organelles that are involved in the breakdown of very-long-chain fatty acids (>18 carbon atoms in length) to medium-chain fatty acids, which are then shuttled to the mitochondrion for further oxidation. Peroxisomal fatty acid oxidation generates 30 per cent more heat than mitochondrial fatty acid oxidation (Baillie et al., 1999). The PPARs are members of the nuclear hormone receptor family, so called because unlike classical hormone receptors, which are located in the cytoplasm and translocate to the nucleus after binding to their ligands, PPARs reside in the nucleus and bind to DNA response elements. The avUCP gene contains a binding site for PPARs in its promoter sequence (Joubert et al., 2010). There are three members of the PPAR subfamily, PPARα, PPARγ and PPARδ, all of which are activated by fatty acids or their derivatives (Clarke et al., 1999). Gene knockout experiments in rodents have verified that UCP3 and UCP2 are not thermogenic whereas UCP1 induces non-shivering thermogenesis (Azzu and Brand, 2009). avUCP does not appear to play a role in thermogenesis in the chicken (Walter and Seebacher, 2009). The distribution of these PPAR isoforms in mammals differs between tissues, and the affinities of activating ligands differs between them (Guri et al., 2006). This may explain why certain fatty acids induce thermogenesis but not UCP expression, why some fatty acids induce thermogenesis but also increase UCP expression and why some fatty acids do not induce thermogenesis but increase UCP expression.
In addition to their effects on UCPs, PPARs also affect the expression of genes for key enzymes in fat and glucose metabolism, which represents another avenue by which cellular responses to heat stress could be manipulated. For instance, heat stress is associated with fatty degeneration of most tissues and the secretion of inflammatory cytokines. Nagasawa et al. (2006) induced hepatic fat accumulation and inflammation in mice by dietary means and showed that pharmacological over-expression of PPARd reduced lipid accumulation and the expression of inflammatory cytokines.
New Oil Seed Varieties Contain Beneficial Fatty Acids
Changes within the oilseed industry brought about by concern about the harmful effects of saturated fatty acids and trans fatty acids have resulted in the development of plant varieties that produce oils high in oleic acid. Saturated fatty acids are converted to the trans configuration by heat during frying and by hydrogenation, used by the industry to improve heat stability for deep-frying or to increase the solidity of oils used for the production of margarine. Trans fatty acids increase cholesterol levels in humans, adding to the incidence of heart disease. As a result of these factors, there is a growing trend away from the use of oils rich in palmitic acid (C16:0) and hydrogenated oils in favour of oils that can provide the required functionality without hydrogenation.
Oils low in palmitic acid and rich in oleic acid, a cis fatty acid, or stearic acid (C18:0) meet these requirements. Oilseed crops such as soybean, rapeseed (canola), peanut, sunflower and cottonseed have now been bred or engineered to produce oil high in oleic acid (Liu et al., 2002). The widespread availability of oils and oil meals derived from high-oleic-acid plants and their increasing incorporation into livestock feeds calls for re-evaluation of the results of trials conducted before the advent of these plants on the use of high-fat diets for heat-stressed poultry.
Conclusion
Recent advances in the pathophysiology of heat stress strongly suggest that the strategic use of new oil seed varieties may ameliorate the adverse effects of heat stress in poultry. A systematic study of the effects of different dietary fatty acids on avUCP expression, PPAR activity and the responses of poultry to heat stress is warranted.
References
Aengwanich W., Sridama P., Phasuk Y., Vongpralab T., Pakdee P., Katawantin S. and Simaraks S. (2003) Songklanakarin Journal of Science and Technology, 25: 297–305.
Aengwanich W. and Simaraks S. (2004) Songklanakarin Journal of Science and Technology, 26: 417–424.
Altan Ö., Pabuçccuoðlu A., Altan A., Knoyalioðlu S. and Bayraktar H. (2003) British Poultry Science, 44: 545–550.
Azzu V. and Brand M.D. (2009) Trends in Biochemical Sciences, 35: 298–307.
Balnave D. (1998) Proceedings of Australian Poultry Science Symposium, 10:34–41.
Balnave D. (2004) Poultry Science, 83: 5–14.
Baillie R.A., Takada R., Nakamura M. and Clarke S.D. (1999) Prostaglandins, Leukotrines and Essential Fatty Acids, 60: 351–356.
Beede D.K. and Collier R.J. (1986) Journal of Animal Science, 62: 543–554.
Bollengier-Lee S., Mitchall M.A., Utomo, D.B., Williams P.E.V. and Whitehead C.C. (1998) British Poultry Science, 39: 106–112.
Butler E.J. (1976) Avian Pathology, 5: 1–14.
Clarke S.D. Thuillier P., Baillie R. and Sha X. (1999) American Journal of Clinical Nutrition, 70: 566–571.
Cronjé P.B. (2005) Recent Advances in Animal Nutrition in Australia, 15: 107–122.
De Lange P., Lanni A., Beneduce L., Morengo M., Lombardi A., Silvestri E. and Goglia F. (2001) Endocrinology, 142: 3414–3420.
Feng J., Zhang M., Zheng S., Xie P. and Ma A. (2008) Poultry Science, 87: 2133–2139.
Geraert P.A. (1998) Proceedings of Australian Poultry Science Symposium, 10: 26–33.
Geraert P.A., Padilha J.C.F. and Guillaumin S. (1996a) British Journal of Nutrition, 75: 195–204.
Geraert P.A., Padilha J.C.F. and Guillaumin S (1996b) British Journal of Nutrition, 75: 205–216.
Guri A.J., Hontecillas R. and Bassanganya-Riera J. (2006) Clinical Nutrition, 25: 871–885.
Hoeks J., Hesselink M.K.C., van Bilsen M., Schaart G., van der Vusse G., Saris W.H.M. and Schrauwen P. (2003) FEBS Letters, 555: 631–637.
Huber J.T., Higginbotham G., Gomez-Alarcon R.A., Taylor R.B., Chen K.H., Chan S.C. and Wu Z. (1994) Journal of Dairy Science, 77: 2080–2090.
Joubert R., Métayer Coustard S., Swennen Q., Sibut V., Crochet S., Cailleau-Audouin E., Buyse J., Decuypere E., Wrutniak-Cabello C., Cabello G., Tesseraud S. and Collin A. (2010) Domestic Animal Endocrinology, 38: 115–125.
Kikusato M., Ramsey J.J., Amo T. and Toyomizu M. (2010) FEBS Letters, 584: 3143–3148.
Lin H., De Vos D., Decuypere E. and Buyse J. (2008) Comparative Biochemistry and Physiology, Part C, 147: 30–35.
Liu Q., Singh S.P. and Green A.G. (2002). Plant Physiology, 129: 1732–1743.
Mahmoud K.Z., Edens F.W., Eisen E.J. and Havenstein G.B. (2004) Comparative Biochemistry and Physiology, Part B, 137: 35–42.
Mahmoud K.Z. and Edens F.W. (2005) Comparative Biochemistry and Physiology, Part C, 141: 69–75.
Manoli I., Alesci S., Blackman M.R., Su Y.A., Rennert O.M. and Chrousos G.P. (2007) Trends in Endocrinology and Metabolism, 18: 190–198.
McKee J.S., Harrison P.C. and Riskowski G.L. (1997) Poultry Science, 76: 1278–1286.
Mujahid A., Toshiki Y. and Toyomizu M. (2005) Poultry Science, 84: 307–314.
Mujahid A., Sato K., Akiba Y. and Toyomizu M. (2006) Poultry Science, 85: 1259–1265.
Mujahid A., Pumford N.R., Botje W., Kakagawa K., Miyazawa T., Akiba Y. and Toyomizu M. (2007a) Journal of Poultry Science, 44: 439–445.
Mujahid A., Akiba Y., Warden C.H. and Toyomizu M. (2007b) FEBS Letters, 581: 3461–3467.
Mujahid A., Akiba Y. and Toyomizu M. (2007c) Poultry Science, 86: 364–371.
Mujahid A., Akiba Y. and Toyomizu M (2009) American Journal of Regulatory, Integrative and Comparative Physiology, 297: R690–R698.
Nagasawa T., Inada Y., Nakano S., Tamura T., Takahashi T., Maruyama K., Yamazaki Y., Kuroda J. and Shibata N. (2006) European Journal of Pharmacology, 536: 182–191.
Njoku P.C. and Nwazota O.U. (1989) British Poultry Science, 30: 831–840.
NRC (1981) Effect of environment on nutrient requirements of domestic animals, National Academy Press, Washington, D.C.
Petracci M., Bianchi M. and Cavani C. (2009) Poultry Science, 88: 1518–1523.
Quinteiro-Filho W.M., Ribero A., Ferraz-de-Paula V., Pinheiro M.L., Saki M., Sá L.R.M., Ferreira A.J.P. and Palermo-Neto J. (2010). Poultry Science, 89: 1905–1914.
Raimbault S., Dridi S., Denjean F., Laucher J., Couplan E., Bouillaud F., Bordas A., Duchamp M., Taouis M. and Ricquier D. (2001) Biochemical Journal, 353: 441–444.
Rey B., Roussel D., Romestaing C., Belouze M., Rouanet J-L., Desplanches D., Sibille B., Servais S. and Duchamp C (2010) BMC Physiology 10:5 (available at www.biomedcentral.com).
Rodríguez V.M., Portillo M.P., Picó C., Macarulla M.Y. and Palou A. (2002) American Journal of Clinical Nutrition, 75: 213–220.
Sahin K., Onderci M., Sahin N., Gursu M.F. and Kuck O. (2003) Journal of Nutrition, 133: 1882–1886.
Sahin K., Onderci M., Sahin N., Gursu M.F., Khachik F. and Kuck O. (2006) Journal of Thermal Biology, 31: 307–312.
Sandercock D.A., Hunter R.R., Nute G.R., Mitchell M.A. and Hocking P.M. (2001) Poultry Science, 80: 418–425.
Sprague J.E., Yang X., Sommers J., Gilman T.L. and Mills E.M. (2007) Journal of Pharmacology and Experimental Therapeutics, 320: 274–280.
Tao X., Zhang Z.Y., Dong H., Zhang H. and Xin H. (2006) Poultry Science, 85: 1520–1528.
Taouis M., De Basilio V., Mognon-Grasteau S., Crochet S., Bouchot C., Bigot K., Collin A. and Picard M. (2002) Poultry Science, 81: 1640–1643.
Thompson M.P. and Kin D. (2004) FEBS Letters, 568: 4–9.
Tripoli E., Giammanco M., Tabacchi G., Di Majo D., Giammanco S. and La Guardia M. (2005) Nutrition Research Reviews, 18: 98–112.
Walter I. and Seebacher F. (2009) Journal of Experimental Biology, 212: 2328–2336.
West J.W. (1999) Journal of Animal Science, vol. 77 and Journal of Dairy Science, vol. 82, suppl 2.








