



A simplified visible light spectroscopic method for the detection and assay of monensin
Monensin is a polyether carboxylic ionophore antibiotic existing as a mixture of four isomers denoted simply as A, B, C and D.Background
The A isomer is the major component (98 percent). It is used for the treatment of coccidiosis in poultry as well as ruminants (cattle, sheep and goats) where it is also used to promote growth.1 Coccidiosis is a parasitic disease which effects a wide variety of species, including poultry, pigs and ruminants. This parasitic infection often causes inappetence, diarrhea, malnutrition, and death through the destruction of the animal’s intestinal lining.2 Monensin is produced commercially as a 40 percent mycelial cake by fermentation using Streptomyces cinnamonensis. The 40 percent mycelial product is further blended with inert ingredients into a variety of products commonly used in the animal health industry. One such product available commercially is marketed by Elanco under the name Rumensin™ 90. It contains 90.7 grams of monensin per pound. In our research we worked with a mycelial product (approximately 40 percent active) obtained from Zhejiang Shenghua Biok Biology Co., Ltd. a Chinese manufacturer located in Zhejiang, P.R., China. Monensin is typically assayed by HPLC. Since Monensin itself is not a chromophore, the HPLC method involves reaction with vanillin in a post column reactor followed by detection of the pink reaction product at 520 nm. As part of a broader, ongoing research project, we wanted to develop a simple method to detect trace amounts of monensin using visible light spectroscopy. Because separation of the various isomers of monensin was not of interest to us, by incorporating the same reagents used in the HPLC method, we were able to quantitatively determine the concentration of monensin by the simple application of a Beer’s Law graph.
When running reaction mixtures of monensin and acidified vanillin in aqueous methanol at 70 C at moderately high molarities (0.025 M monensin, 0.084 M vanillin) the reaction was fairly rapid. At more dilute concentrations however, we noted the absorbance readings of the samples withdrawn at various times from the reaction mixture continuing to increase. We decided to monitor the absorbance of a dilute reaction mixture until it became constant signaling the reaction’s endpoint.
Reagent preparations for Beer’s Law plot of 40 percent Mycelial Monensin
Solutions of Monensin and vanillin were prepared as described below, (Table I). The mycelial monensin sample we obtained was not a uniform particle size and contained both fine powder and larger, lumpy aggregates. It required some mechanical crushing to better facilitate dissolution followed by mechanical agitation using a magnetic stirring bar. The resulting solution was filtered using Whatman 41 ashless paper suspended in a glass funnel which removed any particulate matter remaining in suspension.
Vanillin solution
0.0304 g vanillin (MW 152.15 g/mol) was weighed out and placed in a 100 mL volumetric flask followed by 50 mL of methanol. To this solution, 4 mL of conc. sulfuric acid was slowly added while swirling the flask in an ice bath. When this had cooled and all of the vanillin had dissolved, additional methanol was added to the 100 mL line on the volumetric flask.
Monensin solution
0.1342 g monensin (MW 670.87 g/mol) was weighed out and placed in a 100 mL volumetric flask followed by 50 mL methanol. To this solution, 10 mL of deionised water was added followed by additional methanol to the 100 mL line on the flask. A small magnetic stirring bar was placed in the flask and the solution was allowed to stir for 48 hours at approximately 25 C (room temperature).
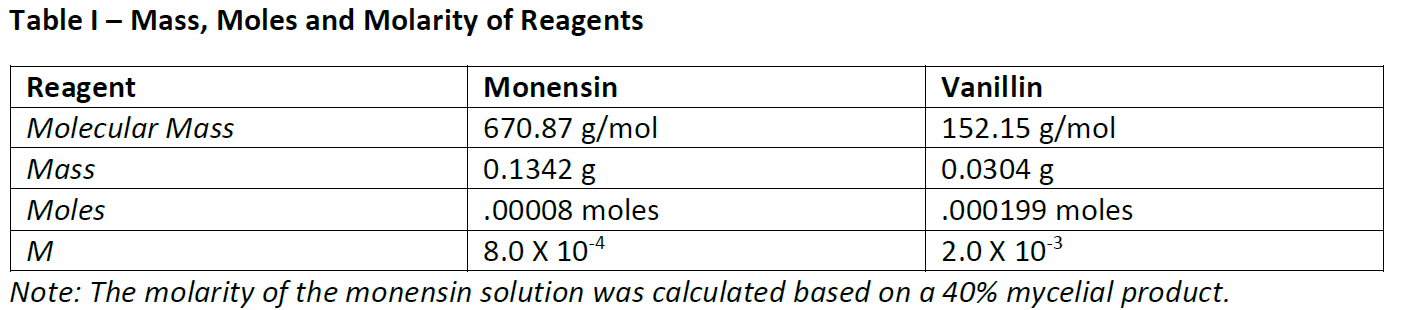
Running the Monensin – Vanillin reaction
50 mL of each solution were combined in a 250 mL Erlenmeyer flask that had been placed in a 70 C water bath. The vanillin solution was clear and colorless. The filtered monensin solution was clear but light brown in color. Prior to mixing, small samples of the individual solutions were withdrawn with a 1 mL micropipette and used to zero the absorbance reading of the spectrophotometer. The reaction mixture slowly developed a pink color which continued to intensify to a cherry red over the course of 90 minutes. 1 mL samples, withdrawn every 5 minutes for the first hour and then every 10 minutes thereafter were placed in cuvettes and their absorbance read after 5 seconds. A graph of absorbance vs. time demonstrated that at 90 minutes the slope of the curve approached zero signifying that the reaction was complete (Graph I).
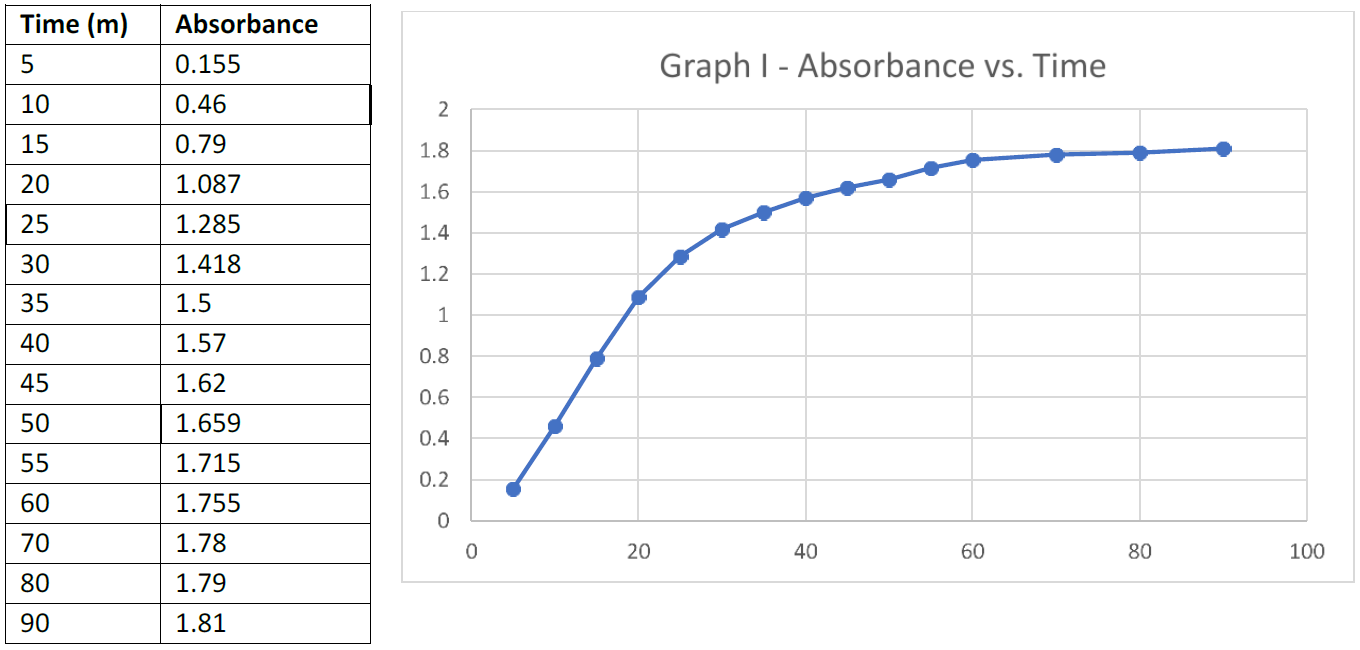
Quantitative assay of Monensin using a Beer’s Law graph
A Beer’s Law plot of absorbance vs. molarity of successively diluted solutions of the reaction mixture after 90 minutes was prepared using Microsoft Excel. Below is the graph showing the results. Also included is the equation for the straight line and the correlation coefficient, (Graph II). A Thermo Scientific Genesys 30 visible spectrophotometer was used to determine all measurements.
Conclusions

As a part of an ongoing undergraduate research project at Palm Beach Atlantic University we wanted to develop a simple method to detect the presence of monensin, monitor the kinetics when reacted with acidified vanillin in dilute solution and quantitatively measure its concentration in the parts per million range. Based on our method we were able to detect monensin in solutions of aqueous methanol using a visible light spectrophotometer at 520 nm to a level of about 3 X 10-6 M, which is approximately 2 mg/L or 2 ppm. In addition to developing this method for our own in-house research, we share it in the hopes that it might be utilised in the animal health industry or at least in a teaching lab at the undergraduate level where an HPLC equipped with a post-column reactor is not available.










