



Avian metapneumovirus update: Epidemiology, Diagnosis, Prevention and Control.
Introduction
The avian metapneumovirus (aMPV) is a respiratory virus that has tropism for the ciliated and non-ciliated respiratory epithelial cells of birds. Replication in these cells causes intracytoplasmic aggregates, abnormalities such as cytoplasmic blebs, loss of cilia and desquamation (Majo et al, 1996)
The histological lesions mentioned above cause a clinical condition in commercial poultry known as swollen head syndrome. It has been shown that inoculation via the oculonasal route in seronegative birds causes respiratory symptoms between 4- and 6-days post inoculation (Cook, 2000). The same author found similar results with intravenous inoculation and described regression or lesions of the oviduct epithelium. These lesions are the cause of a loss of quality and quantity of eggs laid by the birds.
Naylor describes finding similar symptoms with the inoculation of SPF birds with metapneumovirus. In this case, clear or turbid nasal exudate or inflammation of the infraorbital sinuses were observed.
The importance of the synergism generated by the metapneumovirus with bacterial aetiological agents has also been demonstrated. Simultaneous inoculation with aMPV and E. coli by eyedrop and the intranasal route caused an increase in the severity of signs, as well as in macroscopic and microscopic lesions compared to groups inoculated only with aMPV or E. coli separately (Majo, 1997). In another study, an increase in morbidity was observed in the case of dual infection with aMPV and Mycoplasma gallisepticum in turkeys. This dual infection caused a higher incidence in macroscopic lesions compared with the groups given single infections (Naylor, 1992). Regarding Mycoplasma, there have also been recent studies in chicken embryos in which synergy with the metapneumovirus was observed. (Nancy Rüger, 2021)
In another study in turkeys, it was found that the combination of aMPV and ORT in the respiratory tract caused clinical signs with longer persistence of ORT in the respiratory tract and with more severe macroscopic and histological lesions compared to the groups given single inoculations (Maja, 2005).
The aMPV forms part of the respiratory complex that, although it can cause lesions alone, creates a synergy in combination with other aetiological agents such as Mycoplasma gallisepticum. (Sid, 2015)
Epidemiology and diagnosis
Of the 4 subtypes that exist (A, B, C and D) the two that are of interest for poultry farming are A and B, with cross-protection between the two, although subtype B is the most immunogenic (Ball, 2022) and the one that induces better cross-protection (Naylor, 1997)
In a literature review of various epidemiological studies, it was concluded that subtype B is the most widely distributed throughout the world. (Cechinato, 2012; Thunai Al-Shekaili, 2014; Naylor 1997; al hasan, 2022; Abdelmoez, 2019)
There is consensus in the literature on the fact that some cases of aMPV may not show overt clinical signs (Arias, 2015; Cechinato2012) and that apart from the signs not being much in evidence, they are also non-specific and common to many respiratory diseases (Umar, 2016).
It is because of this that an additional method is needed to obtain a correct diagnosis, such as ELISA or PCR. ELISA is the method most widely used (Umar, 2016).
The following concepts must be considered to obtain a correct diagnosis.
In the case of PCR analysis, it is important to understand that the virus remains in the birds’ upper airways for only a short period of time. For this reason, swabs and prints must be taken when symptoms first appear.
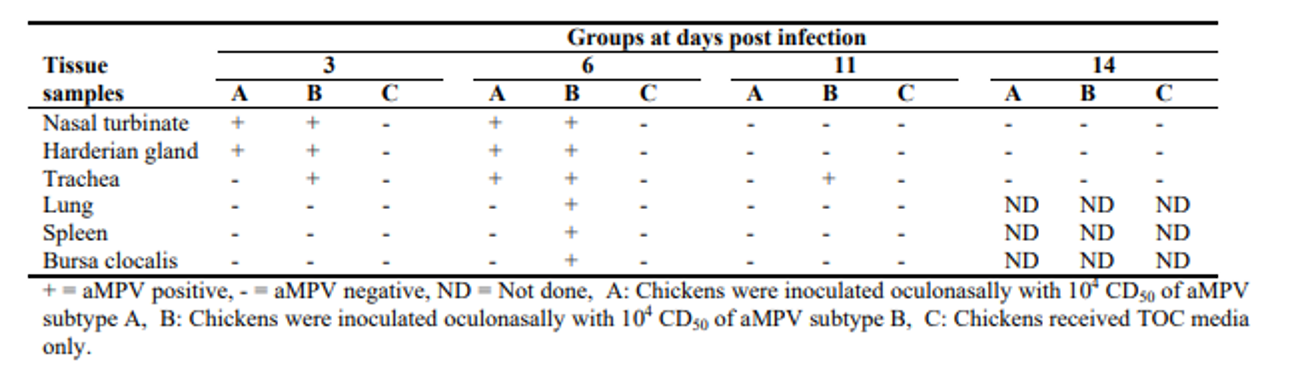
Aung YH., Liman M., Rautenschlein S., 2006. Experimental infection of broilers with avian Metapneumovirus subtype A and B. World's Poultry Science Journal vol. 62, p.134.
As can be seen in the figure (Aung, 2006), the virus can be isolated by PCR in the first six days post inoculation. Other authors were only able to recover the virus by PCR between 3- and 7-days post inoculation (Ball, 2019). Aung was able to detect the first positive ELISA results at 14 days in the case of subtype A and 11 days in the case of subtype B.
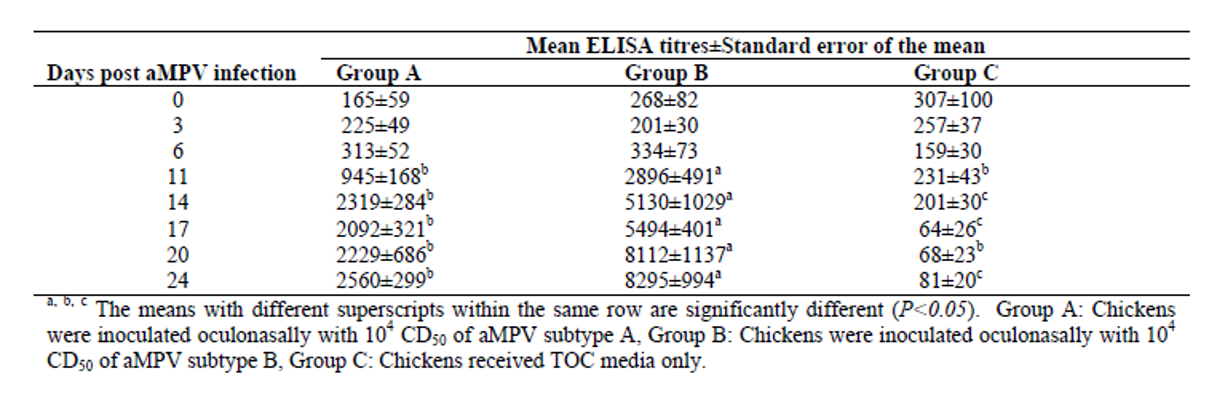
Aung YH., Liman M., Rautenschlein S., 2006. Experimental infection of broilers with avian Metapneumovirus subtype A and B. World's Poultry Science Journal vol. 62, p.134.
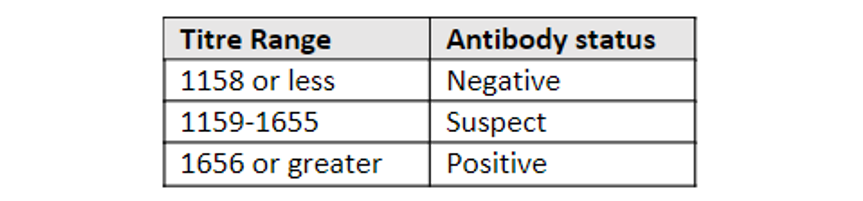
In the case of short life-cycle birds, it is very important to take this information into account in order to avoid false negatives because, if we use the reference values of some commercial kits such as Biocheck as a guide, we will find immunological gaps when there is a negative result by PCR and ELISA.
Backing up this hypothesis, in a field trial in Argentina, PCR and ELISA studies were carried out in parallel at 43 days of age. The results observed were negative serology and PCR positive.
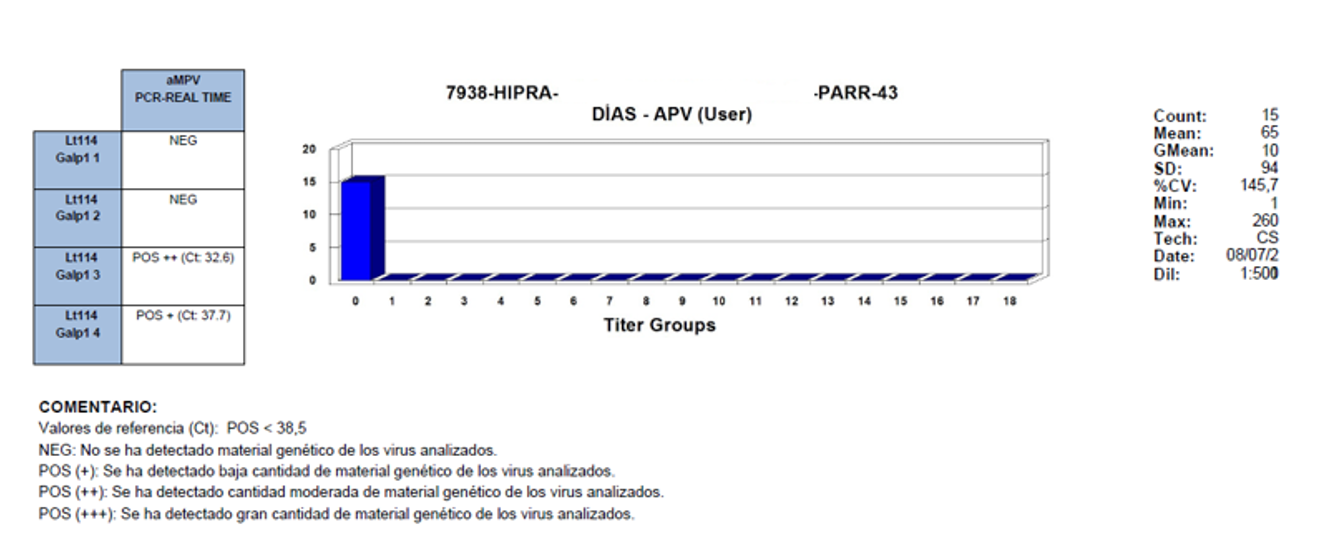
NOTES:
Reference values (Ct): POS < 38.5
NEG: No genetic material of the analysed viruses was detected
POS (+): A small quantity of the genetic material of the analysed viruses was detected
POS (++): A moderate quantity of the genetic material of the analysed viruses was detected
POS (+++): A large quantity of the genetic material of the analysed viruses was detected
The results obtained clearly show that there is immunological silence, the time when there is a delay in the appearance of positive serology in infected birds which, when they are slaughtered at an early age, do not express this positivity.
To facilitate the diagnosis, it is suggested that blood samples should always be taken at slaughter age and, if necessary, a few birds should be retained and blood taken and ELISA carried out again after 15 days, in the expectation of seroconversion.
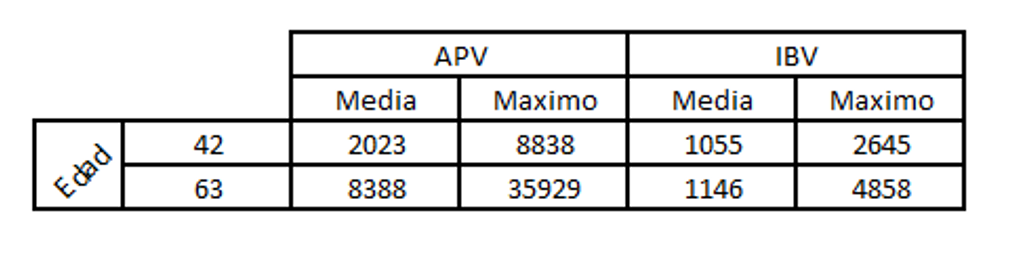
Let us look at a case in which a few short life-cycle birds were showing signs of swollen head and for which an ELISA test was carried out for APV and infectious bronchitis to make a differential diagnosis.
Key to table above:
Edad: age
Media: median
Maximo: maximum
In this case, seroconversion for APV could clearly be seen, but not for IBV.
When serological monitoring is carried out in long life-cycle birds, the situation is very different as, having the necessary time, the birds produce the antibodies which can be detected by ELISA. In these categories, a period of 3 or 4 weeks can be allowed post symptoms before blood samples are taken, or sampling can simply be carried out every 10 weeks. This will facilitate diagnosis.
These concepts presented above are described by different authors (Gharaibeth, 2007; Uriarte, 2010) who found a greater prevalence in long life-cycle birds than in those with a short life-cycle.
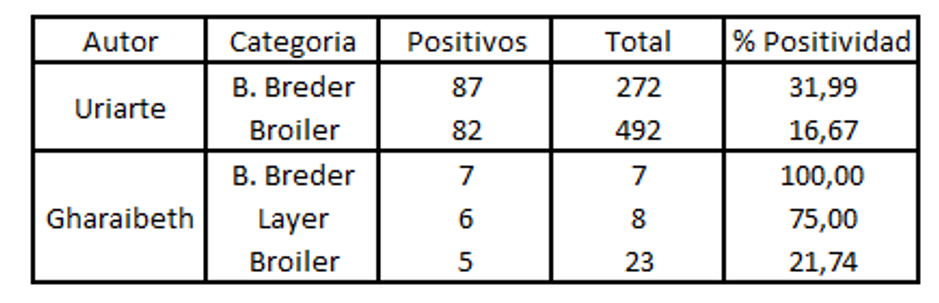
When positivity is analysed by PCR , it is lower compared to that found with ELISA. This is because of the short period for which the virus remains in the upper respiratory tract. (Aung 2006).
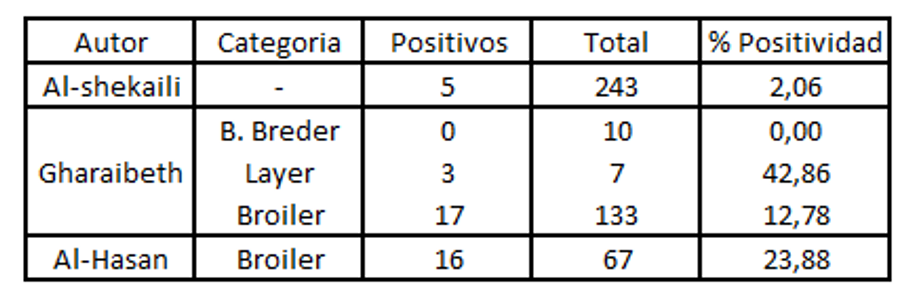
Key to tables above:
Autor: author
Categoria: category
Breder: breeder
Positivos: positive
Positividad: positivity
In the case of broiler chickens, this disease has remained in the background for a long time, because of the prioritisation given to the control of other respiratory diseases such as bronchitis or Newcastle disease, although this is changing for two main reasons:
- Global reduction in the consumption of antibiotics.
- Effective programmes against other respiratory diseases.
Taking this into account, we can state that the metapneumovirus, especially subtype B, is becoming more and more prevalent in broilers (Tucciarone, 2018).
Control and prevention
The APV is an enveloped virus (Loan et al 1992), which means that several disinfectants are effective including quaternary ammoniums, ethanol, phenol derivatives and hypochlorite. The virus is stable at pH 3- pH9 and is inactivated after 30 minutes at 56 ºC (Collins, 1986). It can remain viable after 7 days of drying at room temperature (Townsend, 2000).
Without doubt, an important tool for the control of this disease is vaccination.
For laying birds, a combination of live and inactive vaccines provides complete protection in terms of the quality and quantity of egg production, as well as respiratory symptoms (Cook, 2000).
In the case of broilers, it was demonstrated that the vaccine against the metapneumovirus, given alone or in combination with other respiratory vaccines, conferred protection against challenge compared to the control groups or those which were vaccinated only with a vaccine against bronchitis (Ball 2019).
The combination of the metapneumovirus vaccine with other respiratory vaccines, such as those against Newcastle disease, bronchitis, or bronchitis variants, does not affect the efficacy of any of the vaccines used (Ball 2019).
In broilers, the disease usually takes the form of the appearance of respiratory signs accompanied by secondary bacterial diseases. These usually respond to antibiotic treatment, but the symptoms reappear a few days after the end of treatment. The disease is usually associated with respiratory complexes in which coinfections with bronchitis are seen. In addition, the severity of cases is increasing. (Mernizi, 2022).
In addition to this, when choosing a vaccine for the control of APV, it is important to be clear that subtype B provides better cross-protection against subtype A than vice-versa (Naylor 1997).
Conclusion
Given all of the above, we can state that the avian metapneumovirus is an agent that causes clinical symptoms in birds which vary in severity depending on the degree of interaction with other pathogenic agents, the environmental quality of the sheds, as well as the health status of these. All of which doubtless affects the productive performance of the animals and results in significant economic losses for the farm.
This therefore means that vaccination is necessary, both with live vaccines (for short life-cycle birds) and also with schedules that include live and inactivated vaccines (for long life-cycle birds) for the prevention and control of this aetiological agent, as different studies have provided sufficient evidence of an improvement in the subsequent breeding cycles.
| References | ||||
|---|---|---|---|---|
| Aung YH., Liman M., Rautenschlein S., | ||||
| (2006) | of broilers with avian Rautenschlein S., 2006. Experimental infection Metapneumovirus subtype A and B.. World's Poultry Science Journal | vol. 62, p.134. | ||
| Ball C., Forrester A., Herrmann A., Lemiere S., Ganapathy K. | ||||
| (2019.) | Comparative protective immunity provided by live vaccines of Newcastle disease virus or avian metapneumovirus when co-administered alongside classical and variant strains of infectious bronchitis virus in day-old broiler chicks.. Vaccine. | Dec 10;37(52):7566-7575. | ||
| Ball C., Manswr B., Herrmann A., Lemiere S., Ganapathy K., | ||||
| (2022.) | Avian metapneumovirus subtype B vaccination in commercial broiler chicks: heterologous protection and selected host transcription responses to subtype A or B challenge.. Avian Pathol. | Apr;51(2):181-196. | ||
| Cecchinato M., Lupini C., Ricchizzi E., Falchieri M., MeiniA., Richard CJ., and Catelli E. | ||||
| (2012.) | Italian Field Survey Reveals a High Diffusion of Avian Metapneumovirus Subtype B in Layers and Weaknesses in the Vaccination Strategy Applied.. 1 December; Avian Diseases Digest | 7(4). | ||
| Collins, M.S., R.E. Gough, S.A. Lister, N. Chettle, and R. Eddy. | ||||
| (1986.) | Further characterisation of a virus associated with turkey rhinotracheitis.. Vet Rec | 119:606. | ||
| Cook J., Orthel F., Woods M. A., Orbell S.J., Baxendale W., Huggins M.B., | ||||
| (2000.) | Avian pneumovirus infection of laying hens: Experimental studies,. Avian Pathology, | 29:6, 545-556. | ||
| Gharaibeh SM., Algharaibeh GR. | ||||
| (2007.) | Serological and Molecular detection of avian pneumovirus in chickens with respiratory disease in Jordan.. Poultry Science. | 86: 1677-1681. | ||
| Maja M., Decostere A., Martel A., Chiers K., Froyman R., Nauwynck H. | ||||
| (2005.) | Synergy between avian pneumovirus and Ornithobacterium rhinotracheale in turkeys,. Avian Pathology, | 34:3, 204-211. | ||
| Majó N., Gibert X., Vilafranca M., O'Loan CJ., Allan GM., Costa L., Pagès A., Ramis A., | ||||
| (1997.) | Turkey rhinotracheitis virus and Escherichia coli experimental infection in chickens: histopathological, immunocytochemical and microbiological study.. Vet Microbiol. | Jun 30;57(1):29-40. | ||
| Majó N., Martí M., O'Loan CJ., Allan GM., Pagès A., Ramis A., | ||||
| (1996,) | Ultrastructural study of turkey rhinotracheitis virus infection in turbinates of experimentally infected chickens.. Vet Microbiol. | Sep;52(1-2):37-48. | ||
| Marien M., Decostere A., Martel A., Chiers K., Froyman R., Nauwynck H., | ||||
| (2005.) | Synergy between avian pneumovirus and Ornithobacterium rhinotracheale in turkeys,. Avian Pathology, | 34:3, 204-211. | ||
| Mernizi A., Bouziane S., Fathi H., Criado JL., Bouslikhane M., Ghram A., Catelli E., Mouahid M., Nassik S. | ||||
| (2022.) | First Seroepidemiological and risk factor survey of Avian Metapneumovirus circulation in Moroccan broiler farms.. Veterinarski Glasnik | 2022, 00, 1-18. | ||
| Naylor, C. J., A. R. Al-Ankari, A. I. Al-Afaleq, J. M. Bradbury, and R. C. Jones. | ||||
| (1992) | Exacerbation of Mycoplasma gallisepticum infection in turkeys by rhinotracheitis virus.. Avian Pathol. | 21:295–305. 1992. | ||
| Naylor C., Shaw K., Britton P., Cavanagh D., | ||||
| (1997.) | Appearance of type B avian Pneumovirus in Great Britain.. Avian Pathol | ;26(2):327-38. | ||
| Naylor C., Al‐Ankari A., Al‐Afaleq A., Bradbury J., Jones R., | ||||
| (1992.) | Exacerbation of Mycoplasma gallisepticum infection in Turkeys by rhinotracheitis virus,. Avian Pathology, . | 21:2, 295-305 | ||
| Rüger N., Sid H., Meens J., Szostak MP., Baumgärtner W., Bexter F., Rautenschlein S., | ||||
| (2021.) | New Insights into the Host-Pathogen Interaction of Mycoplasma gallisepticum and Avian Metapneumovirus in Tracheal Organ Cultures of Chicken.. Microorganisms. | Nov 22;9(11):2407. | ||
| Sid H., Benachour K., Rautenschlein S., | ||||
| (2015.) | Co-infection with Multiple Respiratory Pathogens Contributes to Increased Mortality Rates in Algerian Poultry Flocks.. Avian Dis. | Sep;59(3):440-6. | ||
| Townsend, E., D.A. Halvorson, K.E. Nagaraja, and D.P. Shaw. | ||||
| (2000.) | Susceptibility of an avian pneumovirus isolated from Minnesota turkeys to physical and chemical agents.. Avian Diseases | 44:336–342. | ||
| Tucciarone CM., Franzo G., Lupini C., Alejo CT., Listorti V., Mescolini G., Brandão PE., Martini M., Catelli E., Cecchinato M., | ||||
| (2018.) | Avian Metapneumovirus circulation in Italian broiler farms.. Poult Sci. v | Feb 1;97(2):503-509 | ||
| Uriarte J., Suzuki K., Origlia J., Gornatti D., Píscopo M., Cerdá R., Herrero M., Marcantoni H., Unzaga MF., Spinsantti E., Marino F., Pecoraro M., Corva., Petruccelli M. | ||||
| (2010.) | Stochastic estimation of seroprevalence against Ornithobacterium rhinotracheale and avian pneumovirus among chickens in Argentina.. Int J Poult Sci; | 9(4): 352-356. |









