



Campylobacter Studied through the Turkey Production Chain
Although the number of Campylobacter found on the turkey farms were low, this food-borne pathogen was found quite widely on Finnish turkey farms and the slaughterhouse, according to researchers led by Dr Perko-Makela. The highlights of their recently published paper are summarised by Jackie Linden for ThePoultrySite.Background
Päivikki Perko-Mäkelä of the Finnish Food Safety Authority, Evira, and co-authors of the new paper in Acta Veterinaria Scandinavica explain, "Campylobacter is the most common cause of bacterial enteritis worldwide. Handling and eating of contaminated poultry meat has considered as one of the risk factors for human campylobacteriosis. Campylobacter contamination can occur at all stages of a poultry production cycle."
The objective of their study was to examine the the occurrence of Campylobacter during a complete turkey production cycle, which lasts one and half years and started with the parents imported as day-old poults. For the detection of Campylobacter, they compared a conventional culture method with a PCR method. Campylobacter isolates from different types of samples were identified to the species level by a multiplex PCR assay.
The researchers mention that previous work by others found the prevalence of Campylobacter in broiler flocks in Finland to be very low at just 2.9 per cent.
Methods
A total of 456 samples were collected from the turkey parent flock, the hatchery, six different commercial turkey farms and from 11 different stages at the slaughterhouse. For the detection of Campylobacter, a conventional culture and a PCR method were used. Campylobacter isolates (n=143) were identified to species level by a multiplex PCR assay.
Results
The researchers did not detect Campylobacter in samples from the turkey parent flock or the hatchery using the culture method. PCR detected Campylobacter DNA in five faecal samples and one fluff and eggshell sample. Six flocks out of 12 commercial turkey flocks where found negative at the farm level but only two were negative at the slaughterhouse.None of the 150 samples from the turkey parent flock, collected during the rearing and brooding period, and of the 30 samples from the hatchery was Campylobacter-positive either by direct culture or culture following enrichment.
However, using the PCR method, five samples from the parent flock in the brooding farm and one sample from the hatchery were Campylobacter positive.
The PCR products from these samples were sequenced and identified as C. jejuni.
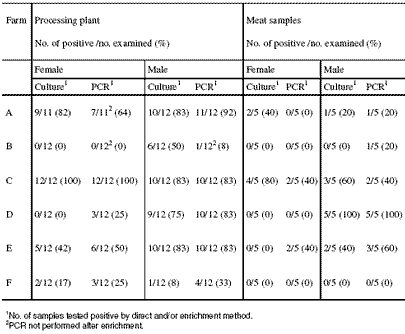
As for the detail, three farms (A, C and E) were found to be colonised with Campylobacter prior to slaughter. At farms A and E, both females and males were found positive. From farm C, only samples from the females were found Campylobacter positive whereas the males were negative at the first sampling. After transport of the females from farm C to the slaughterhouse, the male flock also became colonised with Campylobacter. No Campylobacter were found in the three other farms (B, D and F) either by direct and enrichment culture or by PCR method.
Table 1 shows the percentage of Campylobacter in the flocks at slaughter and at meat cutting. At the slaughterhouse, Campylobacter was isolated from at least one sample in 10 out of the 12 flocks studied. However, from two female flocks of the farms B and D, no Campylobacter was detected. The female flock of farm B was Campylobacter-negative also by PCR method but PCR was not performed after enrichment from the samples of this flock.
Generally, the percentage of Campylobacter of the samples taken during the slaughter process was higher than of those taken during the cutting process. In contrast, the meat samples of the males from farm D were all positive for Campylobacter, while only 75 to 83 per cent of the slaughter samples were positive.
The number of Campylobacter-positive samples taken at the processing plant is shown in Table 2. When using enrichment culture for Campylobacter determination, the highest percentage of positive samples was found in the environmental samples from the evisceration room (75 per cent).
Faecal material collected from the transport crates (67 per cent), the chilling water (67 per cent) and the neck skins (62.5 per cent) had high isolation rates after enrichment. Following enrichment, higher percentages of positive samples were observed among neck skin samples (62.5 per cent) than among the caecal samples (33 per cent).
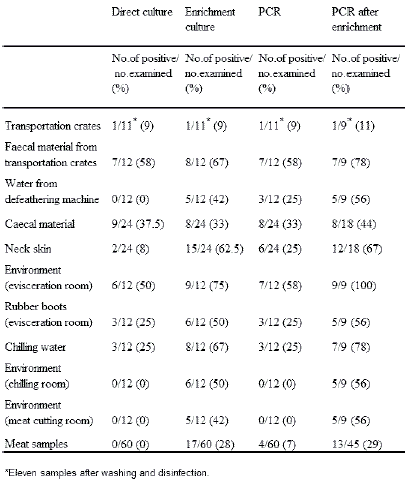
Environmental samples from the chilling- and cutting room were all negative by direct culture and direct PCR. However, following enrichment, 50 per cent and 42 per cent of the same samples from the chilling room, and 56 per cent and 56 per cent from the cutting room were found positive for Campylobacter by culture and PCR, respectively.
Also water samples from the de-feathering machine, neck skin samples, swab samples from the rubber boots of the workers in the evisceration room and meat cutting samples showed a higher percentage of Campylobacter using PCR after enrichment (Table 2).
A total of 143 Campylobacter isolates from samples taken from the commercial farms and the slaughterhouse were identified as Campylobacter spp. by PCR. When species identification was performed using the multiplex PCR method, 105 isolates were identified as C. jejuni and none as C. coli. Thirty-eight isolates were not identified as either C. jejuni or C. coli by the multiplex PCR method. Thirty-four of these isolates originated from different slaughterhouse samples from both female and male flocks from farm C.
The diagnostic specificity for the comparison of direct PCR to direct culture was 0.88 with a level of agreement of 0.88 and for the comparison of both methods by selective enrichment was 0.88 with a level of agreement of 0.92.
Conclusions
* "Reduction of contamination at the farm level by a high level of biosecurity control and hygiene may be one of the most efficient ways to reduce the amount of contaminated poultry meat in Finland." |
The authors says that the presence of Campylobacter DNA from the brooding flock and hatchery sample shows that they have been in contact with Campylobacter but for unknown reasons, the contamination did not appear to have spread.
The present study also shows that during the processing of a Campylobacter-positive turkey flock, working surfaces and equipment at the slaughterhouse can become contaminated, leading to possible contamination of negative flocks, even if slaughtered on subsequent days.
The persistence of Campylobacter on surfaces emphasises the need for efficient cleaning and disinfection of the processing facilities, the Finnish group stressed.
However, the need for enrichment in this study for detection of Campylobacter at certain processing steps, also when performing PCR, might indicate low numbers of Campylobacter at the farm and the slaughterhouse level.
Since complete elimination of thermophilic Campylobacter from the turkey production chain does not seem feasible, the researchers suggest that reduction of contamination at the farm level by a high level of biosecurity control and hygiene may be one of the most efficient ways to reduce the amount of contaminated poultry meat in Finland.
On the subject of the best detection method, Perko-Mäkelä and colleagues noted, "Due to the low numbers of Campylobacter in the Finnish turkey production chain, enrichment PCR seems to be the optimal detection method here."
Reference
Perko-Makela P., P. Isohanni, M. Katzav, M. Lund, M-L. Hanninen and U. Lyhs. 2009. A longitudinal study of Campylobacter distribution in a turkey production chain. Acta Veterinaria Scandinavica, 51:18 doi:10.1186/1751-0147-51-18
Further Reading
| - | You can view the provisional version of the full report by clicking here. |
April 2009








