



Consistency and reliability of the IBD Immune-Complex Vaccine
Learn why balance is so important in an Infectious Bursal Disease (IBD) immune-complex vaccineIt is critical to have a well-defined, balanced antigen-antibody combination to ensure a consistent and reliable induction of IBD immunity in the presence of maternally-derived antibodies (MDA). Specific antibodies cover the IBD virus surface proteins concealing the virus and out of reach from being detected by the immune system.
This immune-complex is sequestered by the dendritic cells and remains there until the passive IBD immunity transferred from the hen to the progeny is catabolyzed and diminishes low enough to allow the virus to be released and fully colonize the bursa. The bursa is now protected against infection from all IBD viruses blocking the re-infection cycle with field IBD viruses.
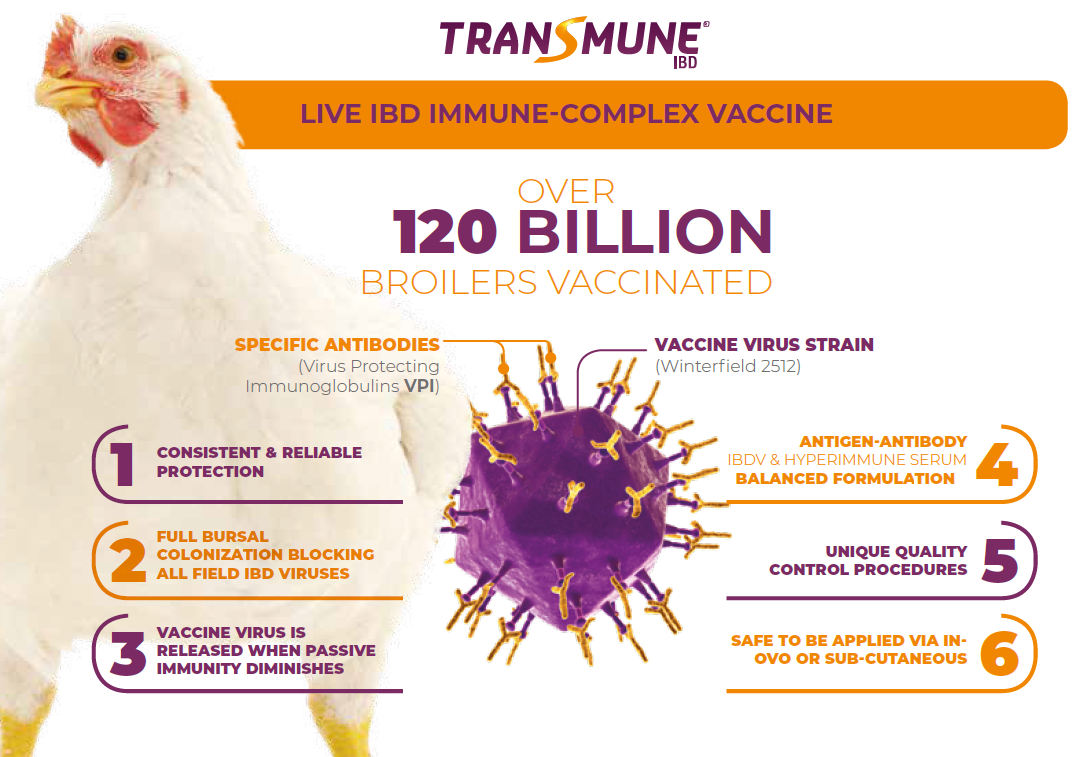
The benefits of this technology are the quality and strength of the protection coming from the IBD virus capability to colonize the bursa without harming the bird, resulting in full IBD protection against clinical signs, complete resistance against IBDV infection, high reduction of shedding and diminishing the risk of virus changes after continuous re-infection. The vaccine adapts to the passive immunity levels of each individual chicken and replicates at the optimum time when MDA levels are at lower level.
Due to the concealing effect of the antibodies covering the vaccine strain in the immune-complex formulation, the vaccine does not get neutralized by MDA. For this reason, the Immune-Complex may be administered in the presence of passive immunity at the hatchery either by In-Ovo or sub-cutaneous routes. The benefits of audited hatchery vaccination ensure the proper immunization of every individual chick. By the beginning of 2021, more than 120 billion birds worldwide were vaccinated with Transmune®, showing the level of confidence and reliability of the poultry industry on this live IBD immune-complex vaccine.
Heterogeneous levels of IBD passive immunity in broiler flocks
Newly placed broiler flocks in a farm are usually composed of progenies from broiler breeder flocks of different ages and IBD immunity levels, in addition to the normal variation in passive immunity levels transferred from hens to the chicks within a single broiler breeder flock. A well-balanced IBD Immune–Complex vaccine must be able to induce immunity facing a very diverse levels of maternally-derived antibodies able to neutralize the IBD virus if not carefully protected. An unbalanced formulation of an immune-complex vaccine may have different effects on a vaccinated flock (See illustration and Table below).
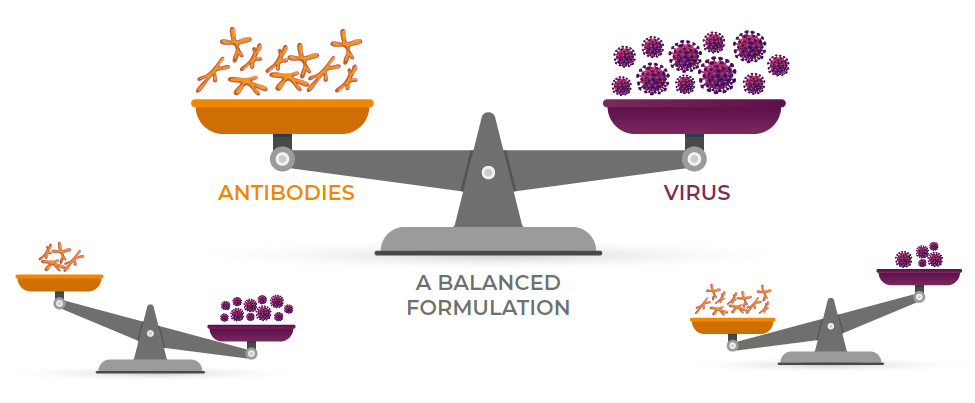
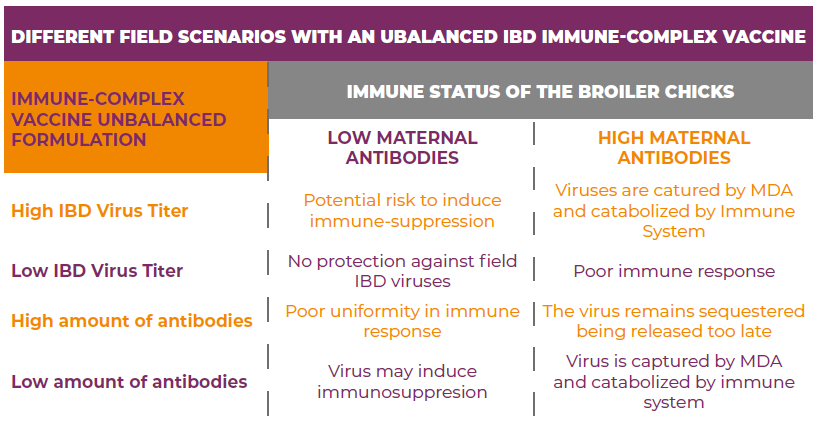
All IBD Immune complex vaccines are different from each other
The immune complex vaccines according to their formulation will have different balance virus- antibody during the vaccine production process, as seen in tests results in the table below.
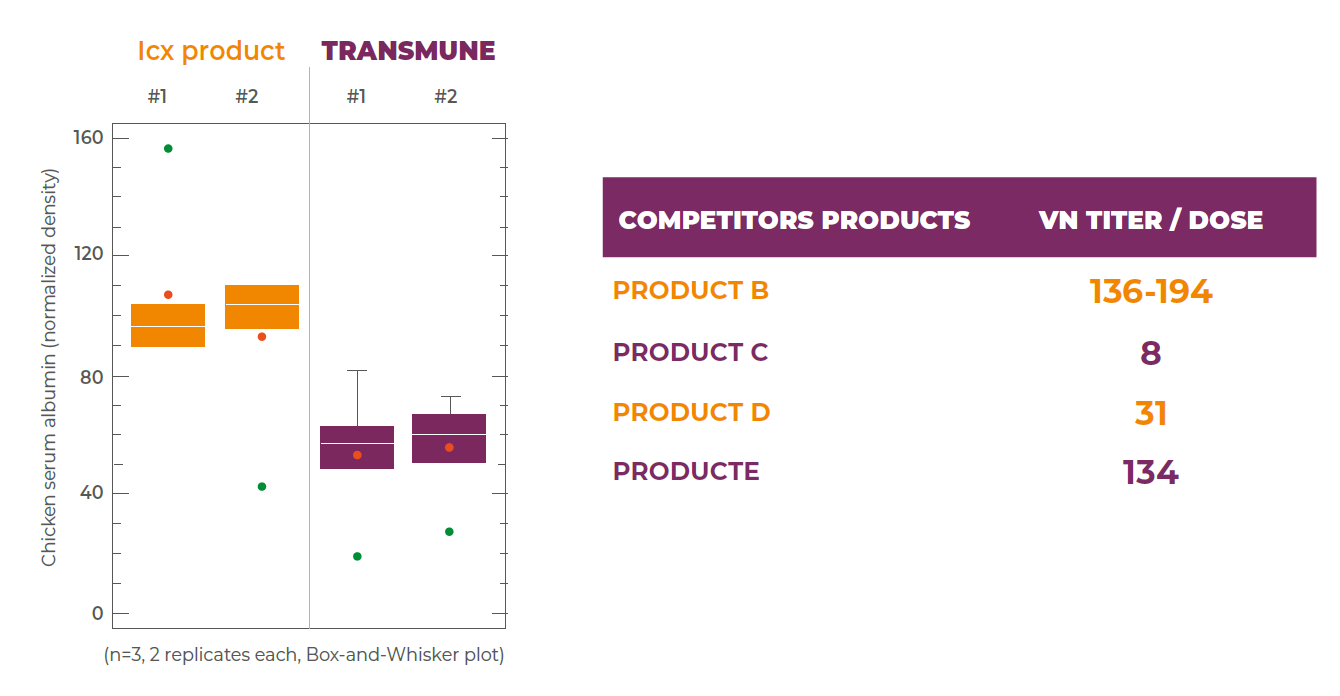
Considering the different in the formulation of different immune complex vaccines is understandable why there are variation of results when they are compared on the poultry farms.
Strict quality control and unique specific potency test (CID50)
Among the differentiating characteristics of the leading IBD immune-complex vaccine (Transmune®) is the unique compliance to the strictest standards of quality, potency and consistency, batch after batch, according to European Regulations.
Vaccine quality includes quality of raw materials and purity of the immunogens without contaminating agents. Additionally, a specific potency test of the final lyophilized Immune-Complex vaccine was uniquely developed to ensure its effectiveness when delivered to the birds vaccinated.
Separate titrations of the IBD virus and the immune serum are not enough quality standards for an immune complex vaccine since both ingredients are eventually mixed before the harsh lyophilization process, where titer losses are implied of the virus and the immune serum in the Immune complex.
The final freeze-dried mixed Immune-Complex product does not grow in embryonated chicken eggs or in cell culture. Ceva scientists had to develop a specific, proprietary protocol to determine the potency of the finished product as an additional quality control test for Transmune®, called CID50 (Chicken Infective dose 50).
Immune-Complex activity is assessed using several biological tests, including bursa histomorphometry and IBD ELISA test to confirm vaccine’s activation. The results are processed statistically using a Model Fit option. A standard has been set for product release and approved by EU regulatory authorities. It means that any batch that would not comply with the established potency standard would be discarded and never brought to the market.
Quality control standards, including the CID50, are another guarantee that our IBD Immune-Complex vaccine (Transmune®) complies with the most stringent quality control standards, batch after batch, and it is a product that customers can rely on.
Biological effects of different IBD Immune-Complex vaccine formulations:
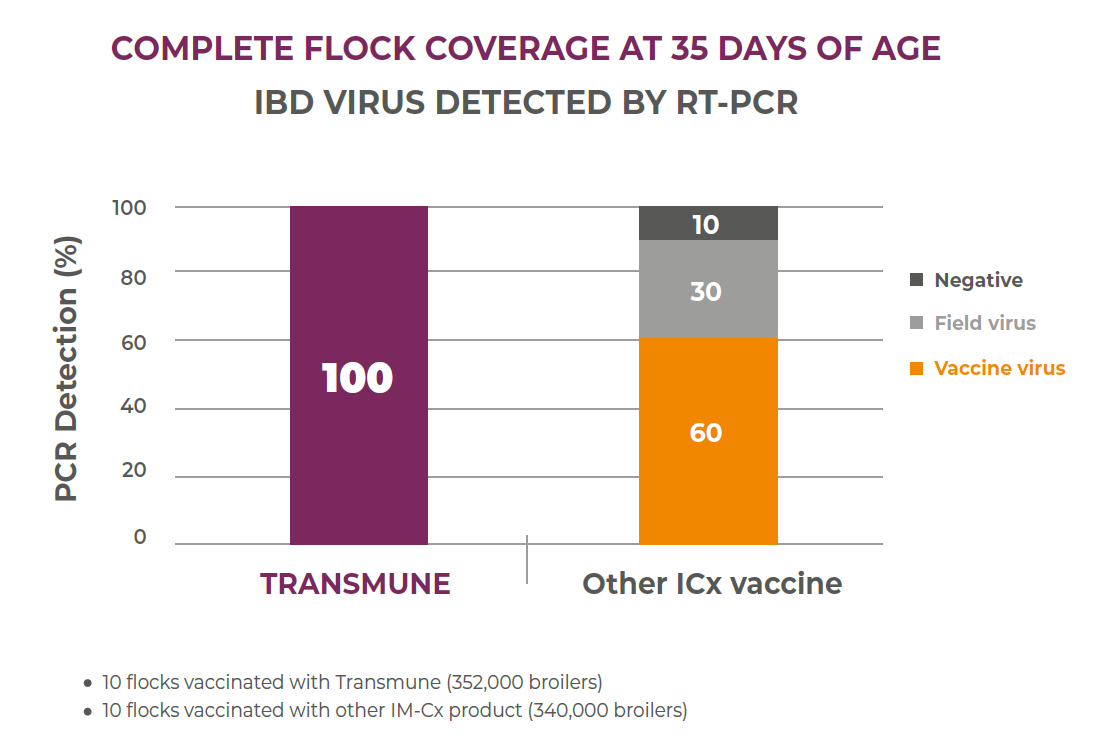
Example 1: A field study in Brazil comparing Transmune with another Immune-Complex vaccine demonstrated the differences in flock coverage at 35 days of age. The comparison included 10 commercial broilers with an estimated population of 352,000 birds (Transmune group) vs. 10 flocks with approximately 340,000 broilers (Other Immune-Complex). Bursa samplings at 35 days of age from each group analyzed by PCR showed complete flock coverage with Transmune in comparison with 60% vaccine detection in the other Immune-Complex vaccine.
Example 2: Another study comparing the IBD vaccine strain detection of four IBD immune-complex vaccines by PCR at different ages of the vaccinated commercial broilers showed the rapid vaccine take of Transmune reaching a 100% at 28 days of age in comparison with the other immune-complex vaccines A, B and C reaching 80%, 60% and 30% respectively at 28 days of age.
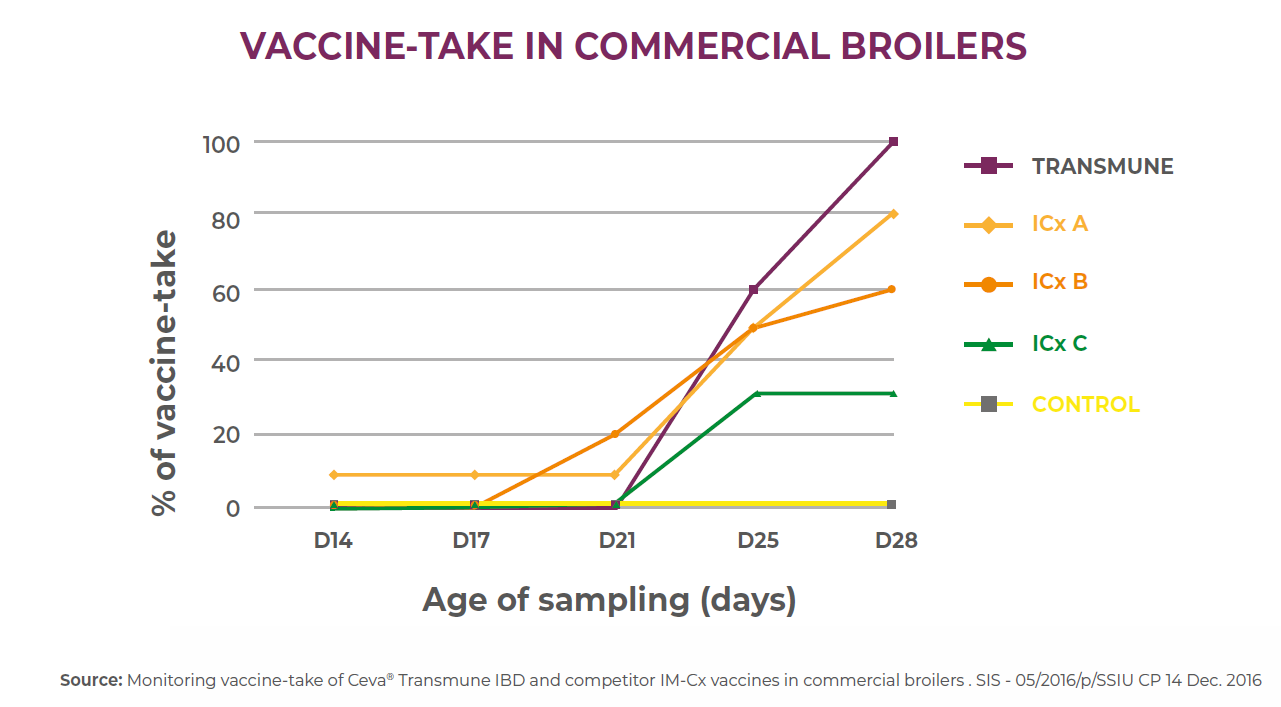
These previous studies showed that Immune-Complex vaccine formulations differences and consequently have different field results in vaccinated flocks.
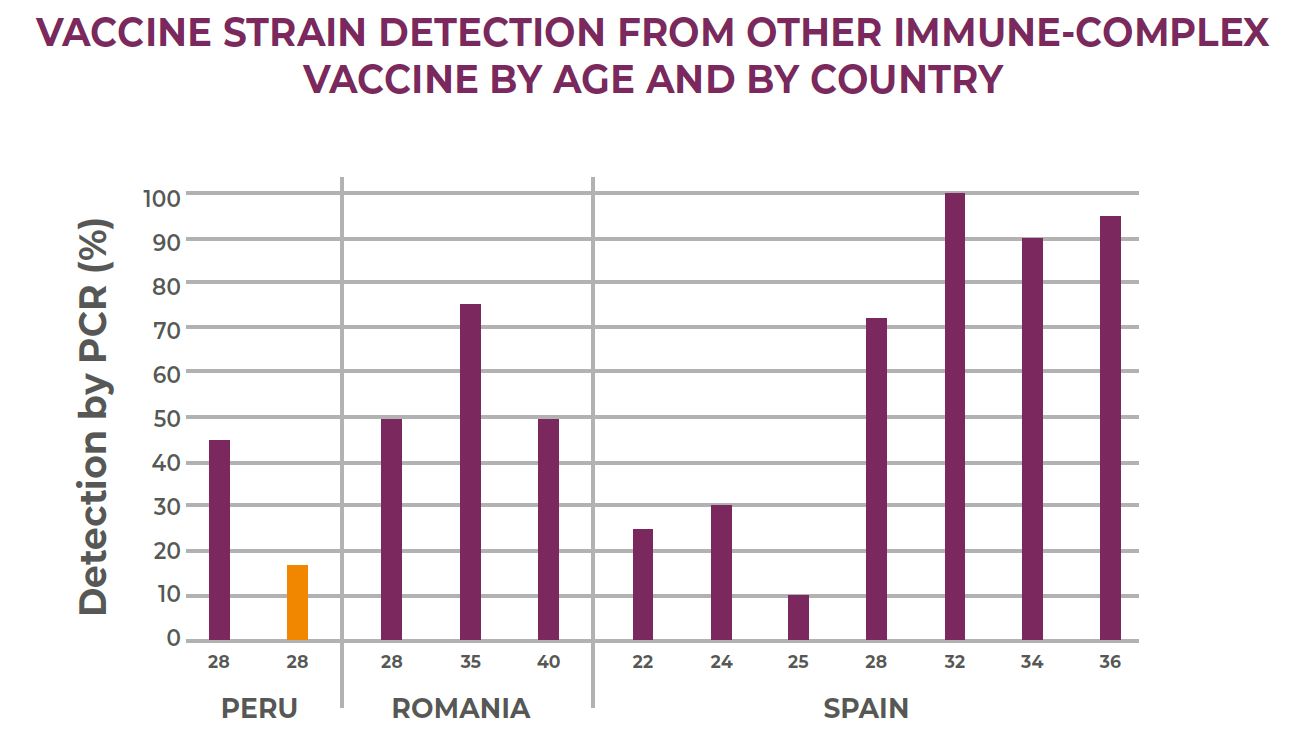
Example 3: Field observations on another Immune-Complex vaccine have shown very variable results on vaccine detection in different countries (See figure below). Possible reasons for these irregular vaccine take may be the level of attenuation of the vaccine strain or the Immune-Complex formulation with low virus content.
Field results on production performance
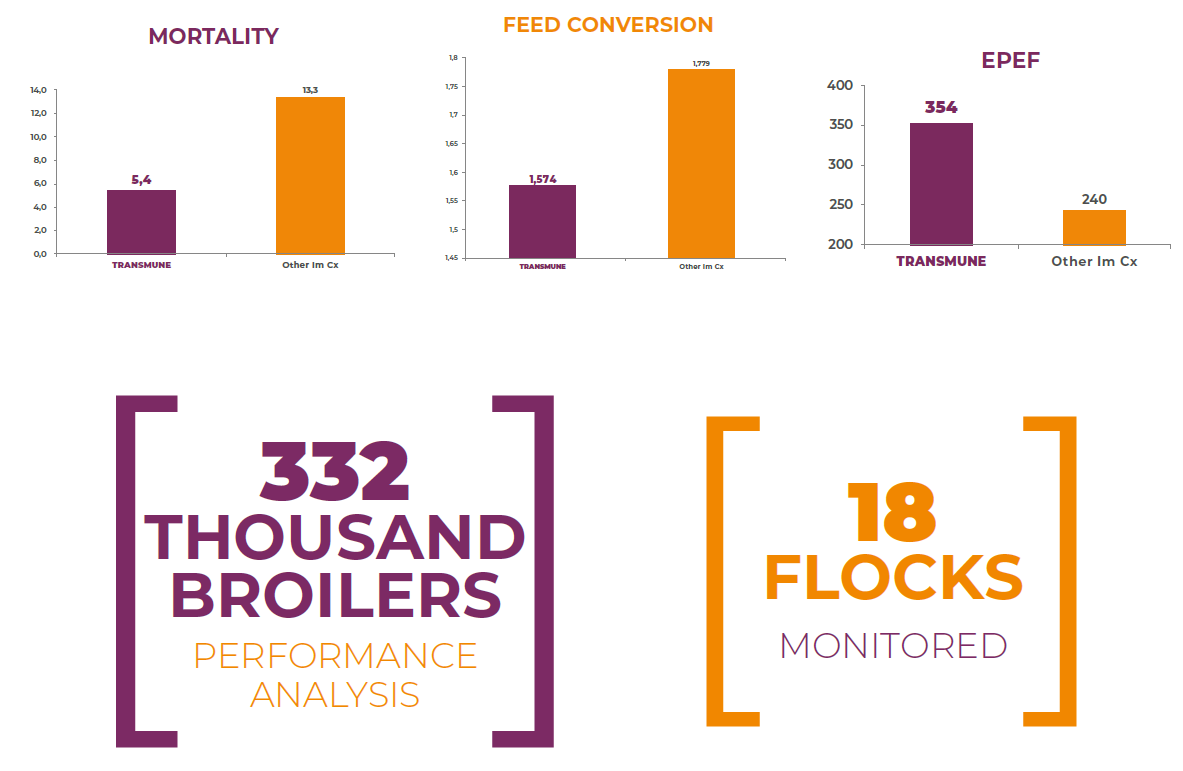
Field Trial # 1 – Egypt (2017): Production performance parameters were compared in a total of 18 flocks comprising 332,000 commercial broilers. These flocks were evenly distributed into two treatment groups. Half of them were vaccinated with Transmune and the other half were vaccinated with other Immune-Complex vaccine. Parameters measured were final body weight, cumulative mortality, feed conversion ratio and European Production Efficiency Factor (EPEF). Comparative results may be observed in the graphic below. The added value of the vaccination with Transmune was estimated into 382 Euros per 1,000 birds’ additional profitability versus de other Immune-Complex vaccine.

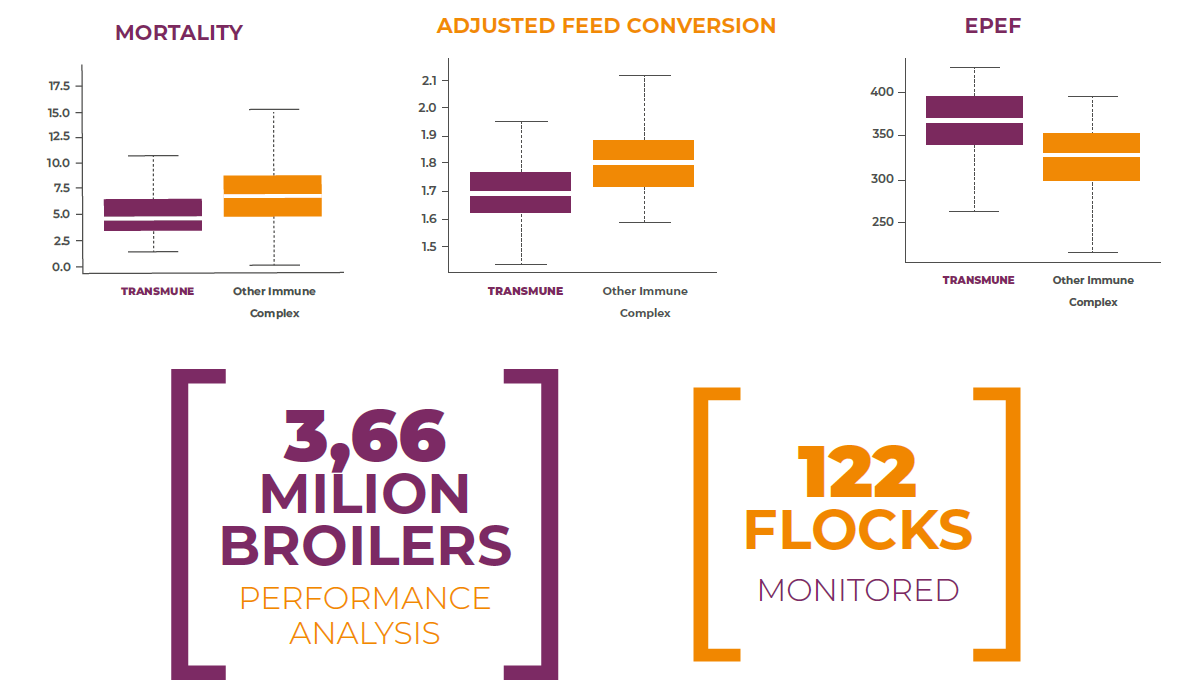
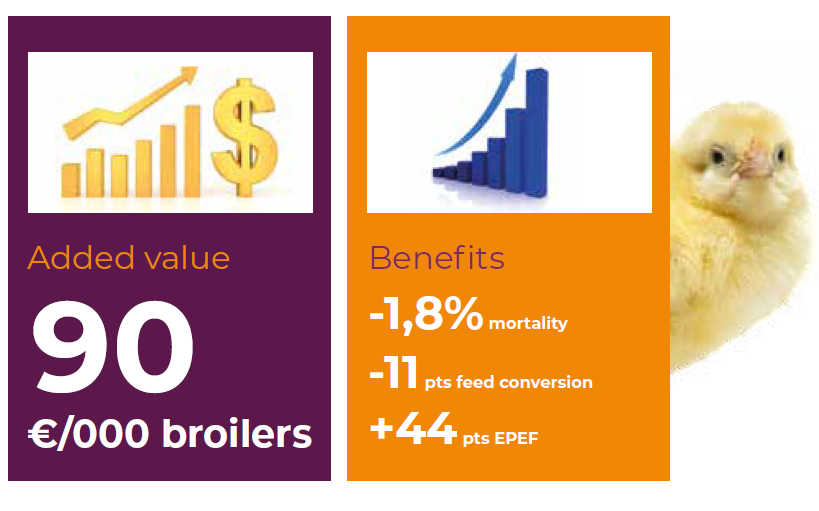
Field Trial # 2 – Venezuela (2018): A field trial conducted in Venezuela in 2018, included a total of 122 commercial broiler flocks comprising over 3.6 million birds. These flocks were distributed into two treatment groups. One half was vaccinated with Transmune and the other half was vaccinated with other Immune-Complex vaccine. Production parameters measured were grow-out period, cumulative mortality, average daily gain, adjusted feed conversion ratio and EPEF. Comparative performance results may be observed in the graphics below. The total added value of the vaccination with Transmune was estimated in 90 Euros additional profit per each 1,000 broilers in comparison with the other immune-complex vaccine.
Summary
- Flock protection and field performance differences have been observed among live IBD Immune-Complex vaccines.
- A balanced formulation Antigen-Antibody is critical for an Immune-Complex vaccine to protect and perform consistently in vaccinated flocks.
- Stringent quality control standards and a unique potency test (CID50) of the final lyophilized Immune-Complex vaccine in Transmune® ensure the consistency and reliability in vaccinated flocks for IBD control.
- Transmune® effectively and safely stops the Gumboro cycle, batch after batch.









