



Construction and Evaluation of Turkey Herpesvirus Vectored Newcastle Disease Vaccine
M. Esaki ¹ ², K. Moore Dorsey ², T. Sato ¹, S. Saitoh ¹, S. Saeki ¹, A. Fujisawa ¹, A. Yasuda ¹ and J. D. Leonard ² ¹ Zeon Corporation, Tokyo, Japan ² Biomune Company, Lenexa, KansasSummary
The fusion gene of Newcastle disease virus was inserted into the genome of a turkey herpesvirus vaccine. Insertion and expression of the fusion gene was confirmed by several molecular assays. The recombinant virus, referred here as rHVT/NDV, was stable after sequential passages in cell culture and specific pathogen free chickens. Safety was demonstrated in chickens as well as multiple avian species. For efficacy studies, the rHVT/NDV vaccine was mixed with the SB1 vaccine strain of Marek’s disease virus and administered in ovo or subcutaneously to day of age chicks. Protection was shown following challenge with the Texas GB strain or the LaSota strain of Newcastle disease virus as well as a very virulent strain of Marek’s disease virus.
Introduction
The etiologic agent of Newcastle Disease (ND) in chickens and turkeys is Newcastle Disease Virus (NDV). NDV is a member of the family Paramyxoviridae, in the subfamily Paramyxovirinae and in the genus Rubulavirus. ND presents itself in many forms ranging from high mortality to an asymptomatic form. There are five forms recognized forms of ND: (1) viscerotropic velogenic Newcastle Disease, (2) neurotropic velogenic Newcastle Disease, (3) Beaudette’s form, (4) Hitchner’ form, and (5) asymptomatic enteric form. NDV strains causing these disease forms are all in one serotype, but are classified into NDV pathotypes based on pathogenicity tests and tissues of virus isolation. Strains are classified as (1) velogenic (high-virulence), (2) mesogenic (moderate-virulence), (3) lentogenic (low-virulence), and (4) asymptomatic.
In an effort to produce an NDV vaccine that protects chicks early in life with one vaccination, we have developed a recombinant vaccine in which the fusion gene from NDV the D26 lentogenic strain of NDV was inserted into the HVT genome. Stability and safety of the recombinant virus was verified by series of experiments. Efficacy of the rHVT/NDV vaccine was demonstrated in specific pathogen free (SPF) chickens and commercial chickens.
Materials and Methods
Newcastle Disease Virus Challenge. NDV challenge was conducted by the intramuscular route using 104.2 EID50/dose of the Texas GB challenge strain of NDV. Chickens were observed daily for 14 days post challenge for neurologic signs such as tremors, loss of coordination, paralysis and death. Respiratory challenge was conducted by the ocular/intranasal route using 103.0 EID50/dose of the LaSota strain of NDV. Efficacy was evaluated by virus reisolation in embryos from tracheal swabs that were taken five days post challenge.
Marek’s Disease Virus Challenge. After challenge with the very virulent RB1B strain of Marek's disease virus by the subcutaneous route to five-day-old chicks, the chickens were observed until seven weeks of age. The chickens were then necropsied and examined for grossly observable lesions consistent with Marek’s disease including, but not limited to, enlargement of the sciatic nerves or tumors (abnormalities) in the kidneys, spleen, liver, heart, gonad, skin or eyes.
Results
The fusion gene cDNA from the D26 lentogenic strain of NDV was obtained and cloned into a homology vector with HVT insertion site sequence (Figure 1). A recombinant HVT was generated by cotransfecting HVT genomic DNA and the homology vector into chicken embryo fibroblast cells and purified by expression of the fusion (F) protein. Insertion of the fusion gene in the purified recombinant virus, rHVT/NDV, was confirmed by the southern blot analysis using an F probe and an HVT insertion site probe, which bind to the fusion gene and the HVT insertion site sequence, respectively (Figure 2).
Also, sequencing of junction regions between the insert sequence and the HVT insertion site sequence was conducted to verify the recombination (data not shown). Expression of the 60-kilodalton F protein was verified by the western blot assay using a rabbit anti-F serum (Figure 3) and a plaque assay using the mouse anti-F monoclonal antibody 3-1G5 (Morrison et al., 1987; data not shown).
Stability of the rHVT/NDV virus was confirmed by sequential passages in cell culture and SPF chickens. The virus recovered after the passages possessed the same gene structure and expressed the 60- kilodalton F protein (data not shown). Safety of the rHVT/NDV vaccine was demonstrated in chickens as well as multiple avian species by a series of tests.
Efficacy of the rHVT/NDV vaccine was evaluated. The rHVT/NDV virus mixed with SB1, rHVT/NDV+SB1, was administered either in ovo to 18-day-old SPF embryos or subcutaneously to day-of-age SPF chicks. At least 90% of vaccinated chickens were protected against challenge with the Texas GB strain of NDV at 5 weeks old (Table 1). Efficacy of the rHVT/NDV+SB1 vaccine against the very virulent RB1B strain of Marek’s disease virus in SPF chickens was also demonstrated (data not shown).
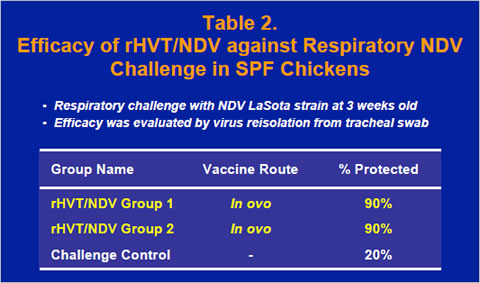
The rHVT/NDV vaccine also provided excellent protection against respiratory challenge with the LaSota strain of NDV in SPF chickens at 3 weeks old. Challenge NDV was isolated from the trachea of only 10% of both vaccinate groups, while challenge virus was reisolated from 80% of the chickens in the challenge control group (Table 2).
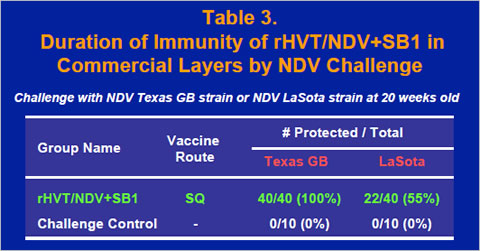
The duration of immunity of the rHVT/NDV+SB1 vaccine was examined by challenging commercial layers at 20 weeks old with the Texas GB strain or the LaSota strain of NDV. All of the vaccinated chickens were protected against the Texas GB challenge, while all of challenge controls showed clinical signs of Newcastle disease or died (Table 3). Also, challenge NDV was isolated from 45% of the vaccinate group, while challenge virus was reisolated from all of the chickens in the challenge control group. Chickens were bled at 5, 10 and 15 weeks old to monitor serological response. ELISA titers of the vaccinates reached ELISA titer of 4000 at 15 weeks old, whereas ELISA titers of non-vaccinated controls stayed less than 200 (data not shown).
The duration of immunity of the rHVT/NDV vaccine was also examined by observing NDV ELISA titers up to 50 weeks old after inoculation by the in ovo route to 18-days-old embryos (Figure 4). After maternal antibodies dropped through the first few weeks, the ELISA titers of chickens vaccinated with the rHVT/NDV vaccine came back up to ELISA titers of 1500-2500 and the high level of anti-NDV antibodies was sustained up to 50 weeks old.
Conclusion
We have constructed a recombinant virus expressing the F protein of NDV. The gene structure and expression of the F protein was verified. Our results showed that the rHVT/NDV virus was stable after sequential passages in cell culture and SPF chickens and safe for use in chickens. The rHVT/NDV vaccine was efficacious against intramuscular challenge with the Texas GB strain of NDV and intraocular/nasal challenge with the LaSota strain of NDV. The duration of immunity was established up to at least 20 weeks old by efficacy against NDV challenge and up to 50 weeks by serological evaluation.









