



Control of Infectious Bursal Disease by an Immune Complex Vaccine
By V. Palya, K. Forgách, T. Süveges, M. Kelemen, J. Mészáros and J. Benyeda CEVA-PHYLAXIA Veterinary Biologicals Co. Ltd., Budapest, Hungary Veterinary Medical Research Institute of the Hungarian Academy of Sciences , Budapest, Hungary PROPHYL Ltd., Mohács, HungaryABSTRACT
The emergency of very virulent infectious bursal disease virus (IBDV) necessiated the development of an infectious bursal disease (IBD) vaccines capable of working in the presence of relatively high level of maternal antibodies. In an attempt to overcome this problem a new vaccine (Cevac Transmune IBD®) which combines an intermediate plus IBD vaccine virus strain complexed with serum against the virus was developed and tested both by in ovo and day-old subcutaneous applications. Vaccinations were carried out on day 18 of embryonic development by in ovo or on the day of hatch by subcutaneous route. The effectiveness of vaccinations was assessed by serological tests, histological examination of bursa and challenge infections performed at different intervals after vaccination. Following vaccination a decline of maternal antibody to IBDV was seen during the first two to three weeks post hatch followed by active humoral immune response before maternal immunity dropped to critically low level. By histological examination, bursal lesions indicative of vaccine virus replication were detected from 14 to 21 days of age onwards in both the in ovo and subcutaneously vaccinated birds. In the challenge experiments non-vaccinated and vaccinated chickens challenged at 14 and 21 days of age were still largely protected by maternal immunity against clinical disease and weight gain depression, however on the basis of bursa pathology the vaccinated animals already exhibited significantly higher protection than the non-vaccinated ones. Following this age, non-vaccinated controls become progressively 100% susceptible to challenge while vaccinates showed full protection from day 28 of age onwards. Our results indicate that immune complex vaccine is an effective means to vaccinate broilers for IBD via a single inoculation and to overcome the problem of variable levels of maternal antibody to IBDV which interferes with conventional live vaccines.
(Key words: broiler, IBD, immune complex vaccine, in ovo vaccination)
INTRODUCTION
Infectious bursal disease (IBD) has been a constant problem for the commercial chicken industry since it’s discovery in the late 1950’s. The only effective way of providing protection against this harmful virus is vaccination of receptive animals at the right time. In young chicks normally variable, but high levels of maternal antibodies persist, that in most cases prevents the traditional live vaccines from inducing active protection after single or even repeated applications. The recently developed IBDV immune complex vaccine prepared by combining live vaccine virus with specific antibody to IBDV (Whitfill et al., 1995) may be applied in ovo or at day old. A great advantage of this type of vaccine that -depending on the maternal antibody level of each vaccinated animal -the vaccine virus starts to replicate at the most appropriate time to provide active immune response to the animals (Sharma et al., 1985, Haddad et al., 1997, Kelemen et al., 2000)
MATERIALS AND METHODS
Vaccine
Cevac Transmune IBD®, an immune complex vaccine that contains specific antiserum mixed in the appropriate ratio with an intermedier plus (Winterfield 2512) IBDV vaccine strain.
Vaccination
18 days old embryonated eggs and day-old broiler chicks posessing maternally derived antibodies to IBDV were used in this study.
Experiment 1: Groups each of 20.000 commercial broiler chickens were vaccinated on day 18 of incubation by in ovo route with 1 dose (0.05 ml) of Cevac Transmune IBD® vaccine or left non-vaccinated. After hatch each group placed into separate chicken houses.
Experiment 2: One day old commercial broiler chicks were divided into two groups of 20.000 each and either left non-vaccinated or vaccinated by subcutaneous route with one dose (0.1 ml/chicks) of Cevac Transmune IBD®. The groups were housed in separate buildings.
Sampling
On day 0, twenty chicks were bled from each group of both experiments to determine the starting maternal antibody titer to IBDV.
On days 7, 14, 21, 28, 35 and 42, twenty chickens from each group of both experiments were bled, euthanised, weighed, necropsied and bursae removed and weighed to determine the bursa / body weight ratios (B/BW) for the calculation of bursa / body weight index (B:B index). Part of each bursa was fixed in buffered formalin and processed for histological examination (haematoxylin-eosin staining) to look for the onset of vaccine virus replication in the bursa.
Virus neutralisation (VN) test
Antibody titer to IBDV was measured by VN test on chicken embryo fibroblast (CEF) cultures against 300500 TCID50 of a tissue culture adopted IBDV strain (GP82). B:B index was calculated according to Lucio formula (Lucio et al., 1979).
Challenge
On days 14, 21, 28, 35 and 42, chickens from each group (n = 20 or 40 / group) were removed to a seperate isolation room and challenged with 1000 EID50 vvIBDV, strain MOM-94 by per os administration. 4 days post challenge, 20 challenged birds from each group were euthanized, necropsied and bursae removed for histological examination. During the necropsy, bursae were scored by gross observation as either susceptible to the challenge (edema and/or haemorrhage) or protected. In the challenge tests carried out at 21, 28 and 42 days of age on chicks from experiment 1, 20 birds per group were weighed individually at the time of challenge and 10 days later again. Assesment of protection was based on the results of the gross pathology and histology of the bursae four days post challenge and on the weight gain during the 10-day period following challenge.
RESULTS AND DISCUSSION
Chicks vaccinated in ovo with 1 dose of Cevac Transmune IBD® hatched normally. The hatching rate of the in ovo vaccinated group (87.1%) did not differed significantly from the one obtained in the non-inoculated control group (86.6%). There was no post hatch mortality exceeding the one occured in the non-vaccinated group. The mortality during the growing period did not differ significantly between the vaccinated and non-vaccinated groups in either of the two experiments (data not shown).
Serology
The day-old chicks had maternal antibody to IBDV of 13.18 and 10.39 log2 in experiment 1 and 2 respectively. Decline of maternal antibody to IBDV was seen during the first few weeks of post hatch reaching its minimum levels (7.45 log2 in the in ovo and 6.62 log2 in the s.c. vaccinated group) between two and three weeks of age. It was followed by active humoral immune response demonstrated by the increase of the antibody titers to IBDV. In both experiments from three weeks of age the antibody levels in the vaccinated groups showed a steady increase while in the non-vaccinated controls the decay of maternal antibodies to IBDV continued (Fig. 1). Chickens having a lower maternal antibody titer to IBDV at hatch (Exp. 2, s.c. vaccinated group) showed an earlier immune response than birds with higher maternal antibodies to IBDV at hatch (Exp. 1, in-ovo vaccinated group). Figure 1. CEVAC Transmune IBD®. Serological response.
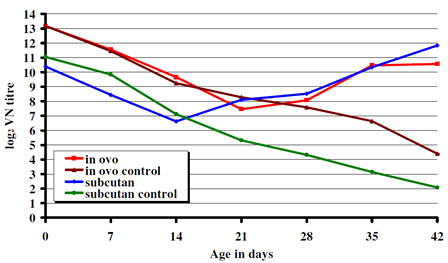
Figure 1. CEVAC Transmune IBD®. Serological response.
Histology and B:B index
In experiment 1, chickens vaccinated in ovo with Cevac Transmune IBD®, bursa leions characteristic of the vaccine virus were detected in 65% of the bursa samples at 21 days of age, while signs of regeneration were already observed by 28 days of age. The mean B:B index of vaccinates was not statistically different from those of non-vaccinates at day 14 of age, indicating that maternal antibody was still high enough to prevent the replication of vaccine virus. However, by 3 weeks of age the mean B:B index (0.52) of vaccinates was statistically lower than those of non-vaccinates, indicating significant vaccine virus replication. By day 28, the mean B:B index reached its minimum and the bursal atrophy in the vaccinated group was apparent. From this age both the bursa histology and the mean B:B index values indicated a progressive regeneration of the bursa. These data demonstrate that the maternal immunity delays vaccine virus replication significantly and also reduces the severity of bursal demage caused by it.
The data from experiment 2 indicated similar evolution of bursa lesions and atrophy (B:B index values) in the vaccinated animals to those recorded in experiment 1. The only significant difference observed was the earlier onset of vaccine virus replication as indicated by the histological lesions and B:B index values. On day 14 of age 70% of the bursae displayed lesions characteristic of vaccine virus which ached 100% with signs of regeneration by day 21 of age This was most probably due to the lower day-old starting maternal antibody level to IBDV. The bursae of the non-vaccinated groups in both experiments remained unaffected during the whole test period.
| Table 1. B:B index | ||||||
| Day/Group | 7th day | 14th day | 21st day | 28th day | 35th day | 42th day |
|---|---|---|---|---|---|---|
| In ovo | 1.16 | 0.95 | 0.52 | 0.28 | 0.34 | 0.45 |
| Subcutan | 1.17 | 0.61 | 0.38 | 0.24 | 0.48 | 0.67 |
The serological and histological results showed that the Transmune IBD® vaccine was not inactivated by the maternal antibodies and resulted in an active immune response as evidenced by the elevated antibody titer to IBDV from 3 to 4 weeks of age onwards. These data demonstrate also that this vaccine can withstand even the neutralizing effect of high level maternal antibodies to IBDV.
Challenge
Chickens from each group of both experiments were challenged on each of days 14, 21, 28, 35 and 42 of age. Based on clinical symptoms and bursa pathology when challenge was carried out at 14 days of age, 60% of the in ovo and 70% of the subcutan vaccinated broilers demonstrated protection against challenge, while the corresponding non-vaccinated controls showed 60% and 100% susceptibility already. At three weeks of age we found 80% of the in ovo and 100% of subcutan vaccinated broilers being protected from challenge, while 90% and 100% of the non-vaccinated corresponding controls were susceptible to the challenge. From 4 weeks of age onwards the vaccinated broilers in both experiments were fully protected from challenge, while all the non-vaccinated animals became fully susceptible (Table 2).
| Table 2. Protection post challenge | ||||
| Age at challenge | In ovo | Subcutan | ||
|---|---|---|---|---|
| Vaccinated | Control | Vaccinated | Control | |
| Protection % | Protection % | Protection % | Protection % | |
| 14 days | 60 | 40 | 70 | 0 |
| 21 days | 80 | 10 | 100 | 0 |
| 28 days | 100 | 100 | 100 | 0 |
| 35 days | 100 | 100 | 100 | 0 |
| 42 days | 100 | 100 | 100 | 0 |
Body weights were measured at the time of challenges and again 10 days later in the challenge experiments carried out at 3, 4 and 6 weeks of age. In the challenge test performed at three weeks of age the mean body weight gain of the vaccinated animals although exceeded, but were not statistically different from the one of the non-vaccinated controls. However, following challenge both at 4 weeks and 6 weeks of age the body weight gains of the vaccinated groups differed significantly (p< 0.0019 and p<0.001, respectively) from the non-vaccinated controls (Fig. 2).
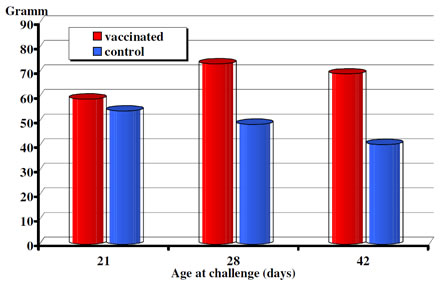
Figure 2. CEVAC Transmune IBD®. Post-challenge daily weight gain (period of 10 days).
The data presented here show that broilers with maternal antibodies to IBDV when receiving a single vaccination either in ovo or subcutaneously at hatch produced active immune response and were fully protected by 3 to 4 weeks of age from challenge. Broilers with higher maternal antibody titers to IBDV at hatch had an active anibody response later then those with moderate levels of starting maternal immunity (Figure 1). The serological results also demonstrate that even very high level of maternal immunity (13.18 log2) did not interfere with the effectiveness of Transmune IBD® in provoking active immune response to IBDV. Both the serological and the bursa pathological (histology, B:B index) results indicate that Transmune IBD® vaccine administered in ovo or s.c. begin to replicate and immunize the chickens before maternal immunity drops to low levels that allows field IBD infection and significant field virus replication (see Fig. 2, influence of challenge on weight gain). Thus, the use of Transmune IBD® vaccine in the hatchery (either in ovo or at hatch) as a single IBD vaccination will narrow the window of susceptibility that often occurs between the loss of maternal immunity and the beginning of active immunity induced by conventional IBD vaccines. This window of susceptibility was either absent or greatly reduced by Transmune IBD® vaccine (Table 2). Furthermore, the use of immune complex IBD vaccine can eliminate the problem of when to vaccinate chickens with different levels of maternal antibody to IBDV and the problem of non-uniform administration of vaccine in the chicken house.
REFERENCES
BENJAMIN LUCIO AND STEPHEN B. HITCHNER (1979) Infectious bursal disease emulsified vaccine: Effect upon neutralizing-antibody levels in the dam. Avian Diseases 23: 466-478
J.M.SHARMA (1985) Embryo vaccination with infectious bursal disease virus alone or in combination with Marek's disease vaccine. Avian Diseaes 29:1155-1169
C.E.WHITFILL (1995) Determination of optimum formulation of a novel infectious bursal disease virus (IBDV) vaccine constructed by mixing bursal disease antibody with IBDV. Avian Diseases 39: 687-699
E.E.HADDAD (1997) Efficacy of a novel infectious bursal disease virus immune complex vaccine in broiler chicken. Avian Diseases 41: 882-889
M.KELEMEN (2000) Pathological and immunological study of an in ovo complex vaccine againts IBD. Acta Veterinaria Hungarica 48: 443-454









