



Controlling Brachyspiras in layers
By David G S Burch, Octagon Services Ltd. and Alan Beynon, BVetMed MRCVS, St David's Poultry Team, Devon - Our knowledge of controlling Brachyspira (spirochaetal) infections in layers is steadily growing. From almost a zero base 2-3 years ago, we are now recognising and increasingly diagnosing the problem, more in free-range flocks than caged layers and occasionally, in replacement pullets.Caged birds seem to respond very well to treatment with tiamulin (Denagard - Novartis) and hygiene methods, including fly and rodent control. Brachyspira pilosicoli, which is the more sensitive spirochaete, has been more commonly associated with cage birds in the UK and causes a 5-6% drop in egg production. It is suspected that it is a combination of the susceptibility of the bacteria to tiamulin and the physical separation from droppings and reinfection that permits such a good response to treatment.
In free-range flocks, the long-term responses to treatment have not been so consistent, although early treatment, around peak production, has given good results in most cases. More flocks seem to be infected with Brachyspira intermedia, 60% in one survey, and this species is less susceptible to tiamulin and often causes a more severe 10-12% drop in egg production. Some flocks respond well, in the absence of the common mycoplasmal, viral and worm problems, but some seem to break down again 3-4 weeks after treatment with another drop in production (see Graph 1).
Graph 1. Repeated drops in egg production associated with reinfection with B. intermedia following treatment (weeks 32, 37 & 41) and prevention (weeks 43-47) with tiamulin
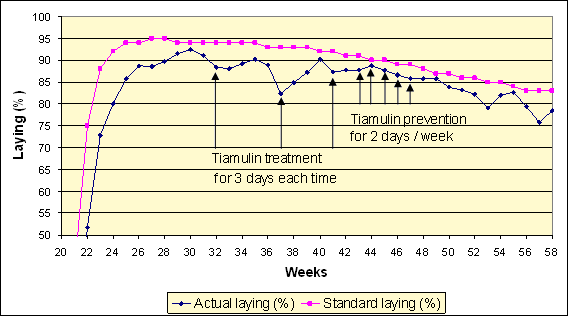
Colonization of the gut can be as short as 5 days, when infection is introduced experimentally but, usually, clinical signs take 7-21 days to develop. In Graph 1, it took 3-4 weeks for the production to fall after treatment, suggesting reinfection was taking place possibly, from the environment or other vectors. According to laboratory trial work, the organism is only meant to survive in faeces for upto 4 days, in cold conditions (see Photo 1), but there is a potential gap in our knowledge, whether it can survive in soil for longer periods. Spirochaetes from pigs have been shown to survive in faeces and slurry for several weeks at 4° C.
 |
| Photo 1. Bare soil outside the hen house - can Brachyspira survive here? |
As a result of the repeated drops in production and the likely reinfection from the environment, a different approach was employed in this flock. Following an initial high dose treatment of tiamulin at week 41, a lower prevention dose was given for 2 days each week (weeks 43-47), to try to prevent the disease from coming back (Burch, 2007). The tiamulin prevention medication worked very well (see Graph1) for the 5 weeks it was given and for a further 5 weeks, but subsequently, production started to drop and become more erratic again. Overall, production was reduced by 5.76% to week 58, in comparison to the breed standard with the biggest drop (week 37) of 10.4%. During the 5-week prevention medication and 5-week follow up (week 43-52) production was only 2.55% lower than the standard
There is still a lot of work that is needed to be done, to finally learn how to control these infections effectively and economically, but progress is being steadily made.
References:
Burch, D.G.S. (2007) Experiences of Avian Intestinal Spirochaetosis in the United kingdom. Proceedings of the 4th International Conference on Colonic Spirochaetal Infections in Animals and Humans Abstract 42.Phillips, N.D., La, T. and Hampson, D.J. (2003) Survival of intestinal spirochaete strains from chickens in the presence of disinfectants and in faeces held at different temperatures. Avian Pathology 32, 6, 639-643.
August 2007 - First published in Poultry World, June 2007








