



Crude Protein 'Requirement' and Maintenance of the Intestine
Low crude protein diets impair broiler performance unless consideration is given to the amino acids required for mucin production and other aspects of gut maintenance, according to Dr E.T. Moran Jr. of Auburn University in the US. He was speaking at this year's Australian Poultry Science Symposium.
Summary
Reduction in feed crude protein (CP) usually follows with dietary inclusion of free essential amino acids (EAA) as a direct means to attain requirements. Although bird EAA needs are assured, reductions in CP eventually impair performance.
Recovery can largely be attained by concomitantly increasing the levels of glycine-serine and proline. Not only are these conditional non-essential (NEAA) difficult to form de novo but prominent in mucin released during small intestinal transit. Membrance-associated mucin (glycocalyx) with enterocytes and goblet cell secretory mucin co-operate to assemble the unstirred water layer (UWL), which acts as a molecular filter of pancreatic digesta accessing the microvilli surface. Large proportions of saccharides O-glycosylated to serine and threonine sterically inhibit mucin proteolysis while glycine and proline give its elastomeric character. The UWL not only protects the surface but associated sugar amination and sulphation act to stabilise the microenvironment. A pH approximating 6.5 appears to facilitate the most favourable nutrient form for membrane transfer.
Given the continuous net loss of constituents associated with mucin, component sourcing for its replacement likely dominates mucosa maintenance. Vascularisation of the villus is structured for immediate access of post-absorptive nutrients to cells at the top which diminishes with flow in the lamina propria to the portal system.
While mucosal EAA needs are implicit in measurement of the bird's requirement, availability of NEAA in proportionate amounts for direct inclusion in mucin facilitates its resynthesis, particularly for serine, glycine and proline. Glutamine is central to N-glucosamine formation and ultimately sialic acid, thus, its presence in appropriate amounts would also be complementary to mucin replacement.
Providing the array of NEAA commensurate with mucin loss when superimposed on needs for EAA is the central hypothesis for a CP 'requirement'.
Mucin/endogenous N loss can vary with lumen conditions; in turn, CP level associated with the feed is expected to follow these changes in order to accommodate the variance in maintenance of the mucosal surface.
Introduction
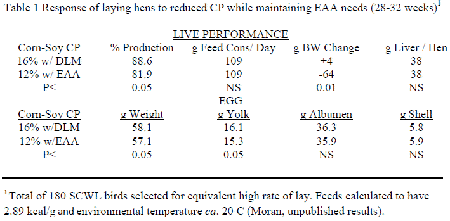
The NRC (1994) indicated that the CP levels given were not to be considered as a requirement of the bird in question but to indicate an amount of N that would be sufficient to form all necessary NEAA. Inclusion of purified forms of each limiting EAA in attaining requirement levels enables a progressive reduction in the feed's CP content. A 'significant' reduction in CP while maintaining EAA and all other nutrients at their minimal needs invariably leads to a loss in live performance. The only known study conducted with laying hens was a short term experiment performed by the author several years ago for another purpose and remains unpublished (Table 1). A small number of Single Comb White Leghorn hens at 28 weeks of age individually selected to be at maximal production were compared when given a 16 per cent CP corn-soybean meal fully adequate feed with one at 12 per cent CP that contained extensive purified EAA to assure requirements. Although both feeds were calculated to be nutritional equivalent, birds receiving the low CP for four subsequent weeks decreased their egg production and lost body weight while eggs also lost weight mainly because of decreased yolk.
Considerable research has been accomplished using the growing bird on reducing CP all resulting in decreased performance and adverse effects on carcass quality. The most recent reports of Delange (2009) and Pesti (2009) indicate that there is no point at which changes become apparent but a continuum occurs without definition. Generally, attempts at fully assuring adequacy and balance of all EAA while maintaining low CP have not been fruitful. Similarly, the inclusion of easy to synthesise NEAA such as glutamic and aspartic acids to increase CP has also been of negligible value (Moran and Stilborn, 1996; Kerr and Kidd, 1999; Bregendahl et al., 2002). However, supplementation of glycine-serine where de novo formation is difficult has proven to be of frequent benefit (Schutte et al., 1997; Dean et al., 2006; Heger and Pack, 1996; Waguespack et al., 2009; Berres et al., 2010).
Combining Conditional NEAA
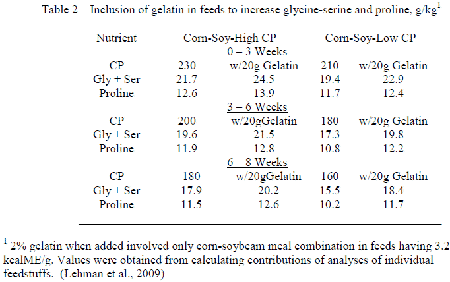
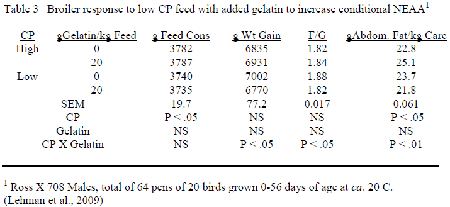
Although the reduction in Low CP with feeds occuring with inclusion free EAA usually decreases the full array of NEAA, resulting change in feedstuffs employed is such that the conditional NEAA decrease to a greater extent than those easily formed. Allen and Baker (1974) evaluated many non-specific N sources in their ability to improve performance of chicks fed purified EAA diets, and only glycine and proline were distinctively better than glutamic acid. Lehman et al. (2009) included gelatin into corn-soybean meal feeds with the broiler such that the levels of glycine-serine and proline increased at low CP to correspond their occurrence when CP with the control corn-soybean diets followed the NRC (1994) recommendation (Table 2). Inclusion of gelatin improved the feed conversion at low CP to agree with that obtained with the control CP having only corn and soybean meal while carcass abdominal fat decreased. The implication is that the loss in conditional NEAA occurring when CP is reduced could not meet the needs to optimise performance.
NEAA and Mucosal Surface
The particularly favourable response of broilers receiving low CP feed to the full array of NEAA where de novo formation is difficult but not those easily transaminated suggests a close and immediate involvement of structural glycoproteins. Birds have an endogenous ileal loss containing substantial amounts of NEAA, particularly glycine, serine and proline (Ravindran and Hendriks, 2004). Mucin loss from the small intestinal mucosa is highly representative in animal endogenous N (Montagne et al., 2000), and inclusion of gelatin in chick feed has been shown to enhance its early development (Fasina et al., 2007). Studies on absorption of all NEAA with swine have demonstrated a minimal entry into the portal system because of an extensive 'consumption' by the surface (Pierzynowski and Sjodin, 1998; Lambert et al., 2006; Bertolo and Burrin, 2008) which is consistent with synthesis of mucin with its return to the lumen.
Understanding the mucosal surface and mucin's function is central to rationalizing need and function of its associated NEAA. Finalisation of digestion-absorption is confined to the upper villus where a mosaic of enterocytes and goblet cells are mutually dependent. Microvilli greatly expand the enterocyte's surface while associated contractile elements facilitate convective exposure. Enzymes such as sucrase, maltase and peptidase anchored in the membrane create concentrated products capable of immediate transfer. Active transport, H+ facilitated transport, and passive movement all exist as appropriate for the nutrient. These activities would be open to ready conflicts from the lumen except for protection by mucins. Essentially, two types of mucins provided by the surface cells co-operate to create the unstirred water layer (UWL).
Microvilli have membrane associated mucins (glycocalyx) that protrude as a fibrous network from the apex. Simplistically, each glycocalyx fibre is anchored in the membrane and linearly projects multiple 'bottle brush-like' domains defined by considerable O-glycosylated short carbohydrate chains to threonine and serine while glycine and proline provide elastomeric character. Secretory mucin is released from goblet cells and has similar bottle brush domains, instead of being linear each is interconnected using cystine to create the equivalent of a free floating net (Bloomfield, 1983). 'Entanglement' of this net with the projecting glycocalyx fibres leads to the UWL, which acts like a molecular filter between the lumen and microvillus surface. The overall strategy with small intestinal digestion is for the pancreatic enzymes to reduce foodstuffs into small units capable of filtering through the UWL for finalisation of digestion. Concurrently, surface enzymes are protected from proteolytic destruction while the products are inaccessible to lumen microbes.
Mucosal Microenvironment
The UWL also appears to provide a stable microenvironment that optimizes the terms for enzymic digestion and molecular form transversing the membrane. Sugars O-glycosylated to mucin approximate six to eight units in length and vary with fucose, galactose, N-glucosamine, N-galactosamine, N-acetyl-glucosamine, N-acetyl-galactosamine, N-acetyl-neuraminic acid (sialic acid) and galactose-6-sulphate being among the possible participants. Not only do these chains sterically hinder proteolysis of the underlying protein chain but their side groups actively participate in stabilising pH of the microenvironment (Shiau et al.,1985). Actual measurements of the human USWL indicated a consistent pH6.5 at the upper villus where active digestion-absorption occurs (Daniel et al.,1985). Changes in the nature of mucin as food consumed changes (More et al.,1987; Sharma et al., 1997) and location along the tract ((Pastor et al., 1988) to suggest that goblet cells can alter the constituent sugars as mucin is produced in order to maintain consistency of pH. Of particular importance, is the glutamine:fructose-6-phosphate amidotransferase as the first and rate limiting step in formation of glucosamine-6-phosphate and all other amino sugars that follow (Li et al., 2007; Durand et al., 2008).
Prolamines in all grains are major contributors of glutamine to feed with 15-20% being provided by zein in corn (Wilson, 1987). Resulting glutamic acid may be used for transamination and formation of other NEAA (Volman-Mitchell and Parsons, 1974; Kight and Fleming, 1995) with the á-keto acid being a major source of energy for the enterocyte (Porteous, 1980; Rhoads et al., 1992; Duee et al., 1995; Wu et al., 1995; Wu, 1998). The advantage of dietary glutamine to mucosal integrity is well established among animals (Tanabe et al., 1963;Yi et al., 2005; Murakami et al., 2007; Zavarize et al., 2008) while representing a substantial amount of dietary NEAA.
Consistency of pH approximating 6.5 in the UWL would appear to enhance the overall digestive absorptive process. Products originating from pancreatic action and chaotic pH in the lumen subsequently assume a favourable 'presentation' upon entering the UWL. Events in the digestion of protein from pepsin through trypsin, chymotrypsin and the carboxypeptidases ultimately lead to EAA being in free form and actively transported while the NEAA end as peptides where their absorption is energized by a 'proton gradient' parallelling the microenvironment (Kan, 1974; Ganapathy and Leiback 1985; Webb, 1990; Kull, 1991). Given their pK values, products of lipid digestion and organic acids have minimal charge conflict and may avail themselves of Na+ for membrane transit (Wolffram et al., 1992). Furthermore, villi enterocytes not only have the ability to modify surface enzymes finalizing digestion according to need (Kushak et al., 1981; Ozols and Sheshukova, 1984) but these enzymes near maximize their activities corresponding to the microenvironment pH (Mizuno et al., 1982; Jamadar et al., 2003). Thus, maintaining the extent and integrity of the UWL is of primary importance to nutrient recovery from the small intestinal lumen.
Mucin Turnover and Crude Protein
Mucin lost from the surface can vary markedly. Lehr et al. (1991) estimated the rat's mucosal mucus gel layer to have a turnover time in the range of 47 to 270 minutes. The low value is envisaged to represent the secretory mucin that is released in free form and frequently from goblet cells, whereas the high value is more likely to be membrane associated mucin of the enterocyte's glycocalyx and more refractory to loss. Overall ability of the pancreatic enzyme complement to erode mucin is expected to be far less than capabilities of microflora within the lumen. Conventional birds have greater villus area and cellular migration rate than if germ-free that demands corresponding additional mucin (Cook and Bird, 1973; Muramatsu et al., 1987). Goblet cells alter the nature of mucin in response to microbial load (Forder et al., 2007); however, rate of mucin degradation appears to be limited until entry into the large intestine and anaerobic terms necessitate access to the 'bottlebrush' CHO complex (Salter and Fulford, 1974; Parsons et al., 1983).
Increased microbial population, reduced partial pressure of oxygen from mucosal transfer, and facilitation of facultatives contribute to the adverse effects of feedstuffs creating viscosity of lumen contents. Clostridia are opportunistic in this respect while having the fucosidases, neuraminidases and proteases to collapse mucin structure (Wold et al., 1974; Chow and Lee, 2007; Olkowski et al., 2008), particularly with surface disruptions created by coccidia (Baba et al., 1997; Pederson et al., 2008). The shift from neutral to acidic type mucin by goblet cell when confronted by Clostridium perfringens can be interpreted as an attempt to maintain the most favourable pH of a compromised microenvironment (Golder et al., 2010). Decreasing the level of CP concurrent with mucosal assaults arising from an adverse microbial population measurably accentuates the complications (Welch et al., 1986; Waldenstedt et al., 2000; Drew et al., 2004; Dahiya et al., 2007).
Relative inability to recover the components comprising mucin originating from either extruded cells or eroded from the surface necessitates its complete regeneration using 'new' nutrients. Villus vascularisation is such that the 'ease' of mucin replacement is dependent on the nutrients absorbed at that time and restricted to the immediate area. Essentially, the mesenteric arteriole terminates at the villus apex then it subdivides into many venules that descend within the lamina propria adjacent to all surfaces (Aharinejad et al., 1991). Oxygen immediately supports active absorption at the top then recovered nutrients may be employed for cell differentiation and repair with their progression to the portal system at the base. Threonine invariably represents the most limiting EAA and variation in its measurement of need follows microbial load and mucin dynamics (Kidd et al., 2003; Horn et al., 2009). Although all NEAA can be synthesized, their access in direct form and proportions relieves the cells in obtaining a source of N, delayed presence and extended work to do so. Absence of glucosamine and other sugar amines also dictates a need for NEAA, specifically glutamine.
Crude protein requirement likely represents need by the mucosa for NEAA and immediate formation of mucin. Once formed, mucin enters the lumen and ultimately becomes part of the endogenous N loss. Kamisoyama et al. (2010) measured the true amino acid digestibilities (TAAD) of adult roosters given low through progressively increasing levels of CP. Reductions of TAAD for aspartic acid, threonine, glutamic acid, proline, glycine, valine, methionine, and isoleucine were observed at the mid-jejunum for the low CP feed that progressively increased with CP level. Prioritisation of mucin formation and maintenance of the mucosa were likely the basis of decreases in amino acid 'availability' when CP was low rather than inadequate digestion. Corrections using endogenous N loss include all amino acids associated with mucin. These amino acids have been 'productively used' to maintain the mucosa; thus, their inclusion for 'correction' with other N sources that are undigestible seems inappropriate to the objective.
References
- Aharinejad S., Lametschwandtner A., Franz P. and Firbas W. (1991) Scanning Microscopy 5, 811-849.
- Allen N.K. and Baker D.H. (1974) Poultry Science 53, 258-264.
- Baba E., Ikemoto T., Fukata T., Sasai K., Arakawa A. and McDonald L.R. (1997) Veterinary Microbiology 54, 301-308.
- Berres J., Vieira S.L., Dozier W.A. III, Cortes M.E.M., deBarros R., Nogueira E.T. and Kutschenco M. (2010) J. Applied Poultry Science 19, 68-79
- Bertolo R.F. and Burrin D.G. (2008) Journal of Nutrition 138, 2032S-2039S.
- Bloomfield V.A. (1983) Biopolymers 22, 2141-2154.
- Bregendahl K., Sell J.L. and Zimmerman D.R. (2002) Poultry Science 81, 1156-1167.
- Chow W.L. and Lee Y.K. (2008) British J. Nutrition 99, 449-454.
- Cook R.H. and Bird F.H. (1973) Poultry Science 52, 2276-2280.
- Dahiya J.P., Hoehler D., Van Kessel A.G. and Drew M.D. (2007) Poultry Science 86, 2358-2366.
- Daniel H., Neugebauer B., Kratz A. and Rehner G. ((1985) American J. Physiology 248, G293-G298
- Dean D.W., Bidner T.D. and Southern L.L. (2006) Poultry Science 85, 288-296.
- Delange L.L. (2009) Australian Poultry Science Symp. 21, 1-8.
- Drew M.D., Syed N.A., Goldade B.G., Laarveld B. and Van Kessel A.G. (2004) Poultry Science 83, 414-420.
- Duee P-H., Darcy-Vrillon B., Blachier F. and Morel M-T. (1995) Proceedings of the Nutrition Society 54, 83-94.
- Durand P., Golinelli \_Pimpaneau B., Mouilleron S., Badet B. and Badet-Denisot M-A. (2008) Archives of Biochemistry and Biophysics 474, 302-317.
- Fasina Y.O., Moran E.T., Ashwell C.M., Conner, D.E.., Leslie M. and McKee S.R. (2007) International J. Poultry Science 6, 944-951.
- Forder R.E.A., Howarth G.S., Tivey D.R. and Hughes (2007) Poultry Science 86, 2396-2403.
- Ganapathy V. and Leiback F.H. (1985) American J. Physiology 249, G153-G160.
- Golder H.M., Geier M.S., Hynd P.I., Forder R.E.A., Boulianne M. and Highes R.J. (2010) Proc. Australian Poultry Symposium 21, 211-214.
- Kerr B.J. and Kidd M.T. (1999) J. Applied Poultry Research 8: 298-309.
- Heger J. and Pack M. (1996) Agribiological Research 49, 257-263.
- Horn N.L., Donkin S.S., Applegate T.J. and Adeola O. (2009) Poultry Science 88, 1906-1914.
- Jamadar V.K., Jamadar S.N., Dandekar S.P. and Harikumar P. (2003) J. Food Science 68, 438-443.
- Kamisoyama H., Honda K., Kubo S. and Hasegawa S. (2010) Japanese Poultry Science 47, 220-226.
- Kidd M.T., Barber S.J., Virden W.S, Dozier W.A. Jr, Chamblee D.W. and Wiernusz C. (2003) J. Applied Poultry Research 12, 115-123.
- Lehr C-M., Poelma F.G.J., Junginger H.E. and Tukker J.F. (1991) International J. of Pharmaceutics 70, 235-240.
- Kan C.A. (1974) Worlds Poultry Science J. 31, 46-56.
- Kight C.E. and Fleming S.E. (1995) Nutritional Biochemistry 6, 27-37.
- Kull F.J. (1991) Biochemical Archives 7, 245-247.
- Kushak R., Ozols A., Antonyuk Z., Tarvid I., Sheshukova T. and Nasurlaeva I. (1981) Comparative Biochemistry Physiology 70A, 107-109.
- Lambert B.D., Filip R., Stoll B., Junghans P., Derno M., Hennig U., Souffrant W.B., Pierzynowski S. and Burrin D.G. (2006) Journal of Nutrition 136, 279-2784.
- Lehman R., Moran E.T. Jr and Hess J.B. (2009) Poultry Science 88, 984-993.
- Li Y., Roux C., Lazereg S., LeCaer J-P., Laprevote O., Badet B. and Badet-Denisot M-A. (2007) Biochemistry 46, 13163-13169.
- Mizuno K., Moriuchi S. and Hosoya N. (1982) J. Nutrition Science Vitaminology 28, 599-608.
- Montagnel Toullec R., Formal M. and Lalles J.P. (2000) J. Dairy Science 83, 2820-2828.
- Moran E.T. Jr and Stilborn H. (1996) Poultry Science 75, 120-129.
- More J., Fioramonti J. and Bueno L. (1987) Histochemistry 87, 189-194.
- Muramatsu T., Takasu O., Furuse M., Tasaki I. and Okumura J-I. (1987) Biochemistry J. 246, 4575-479.
- Murakami A.E., Sakamoto M.I., Natali M.R.M., Souza L.M.G. and Franco J.R.G. (2007) Poultry Science 86, 488-495.
- NRC (1994) Nutrient Requirements of Poultry. Ninth Revised Edition edited by National Academy Press, Washington, D.C. USA.
- Olkowski A.A., Wojnarowicz C., Chirino-Trejo M., Laarveld B. and Sawicki G. (2008) Research in Veterinary Science 85, 543-553.
- Ozols A. and Sheshukova T. (1984) Comparative Biochemistry Physiology 77B, 636-637.
- Parsons C.M., Potter L.M. and Brown R.D. Jr (1983) Poultry Science 62, 483-489.
- Pastor L.M., Ballesta J., Madrid J.F., Perez-Tomas R. and Hernandez F. (1988) Acta Histochemica 83, 91-97.
- Pederson K., Bjerrum L., Heuer O.E., Lo Fo Wong D.M. and Nauerby B. (2008) Avian Diseases 52, 34-39.
- Pesti G.M. (2009) Poultry Science 88, 477-476.
- Pierzykowski S.G. and Sjodin A. (1998) J. Animal and Feed Sciences 7, 79-91.
- Porteous J.W. (1980) Biochemistry J. 188, 619-632.
- Ravindran V. and Hendriks (2004) Animal Science 79, 265-271.
- Rhoads J.M., Keku E.O., Woodard J.P., Bangdiwala S.I., Lecce J.G. and Gatzy J.T. (1992) American J. Physiology 263, G960-G966.
- Salter D.N. and Fulford R.J. (1974) British J. Nutrition 32, 625-637.
- Schutte J.B., Smink W. and Pack M. (1997) Archiv fur Geflugelkunde 61, 43-47.
- Sharma R., Fernandez F., Hinton M. and Schumacher U. (1997) Cellular and Molecular Life Sciences 53, 935-942.
- Shiau Y-F., Fernandez P., Jackson M.J. and McMonagle S. (1985) American J. Physiology 248, G608-G17.
- Tanabe S., Watanabe M. and Arai S. (1993) J. Food Biochemistry 16, 235-248.
- Volman-Mitchell H. and Parsons (1974) Biochimica et Biophysica Acta 334, 316-327.
- Waguespack A.M., Powell S., Bidner T.D. and Southern L.L. (2009) J Applied Poultry Research 18, 761-765.
- Waldenstedt L., Elwinger K., Lunden A., Thebo P. and Uggla A. (2000) Archiv fur Geflugelkunde 64, 34-39.
- Webb K.E. (1990) J. Animal Science 68, 3011-3022.
- Welch C.C., Parsons C.M. and Baker D.H. (1986) Poultry Science 65, 1939-1944.
- Wilson C.M. (1987) pp 273-310 in Corn Chemistry and Technology, edited by Watson SA and
- Ramstad P.E., American Association of Cereal Chemists, St Paul, MN.
- Wold, J.K., Midtvedt and Jeanloz R.W. (1974) Acta Chemica Scandinavica B 28, 277-284.
- Wolffram S., Hagemann C., Grenacher B. and Scharrer E. (1992) Comparative Biochemistry Physiology 101A, 759-767.
- Wu G. (1998) J. Nutrition 128, 1249-1252.
- Wu G., Flynn N.E., Yan W. and Barstow D.G. Jr (1995) Biochemistry J. 306, 717-721.
- Yi G.F., Allee G.L., Knight C.D. and Dibner J.J. (2005) Poultry Science 84, 283-293.
- Zavarize K.C., Sartori J.R., Pelicia V.C., Pezzato A.C. and Carrijo A.S. (2008) Proceedings World's Poultry Science Meeting, Brisbane, Australia.
Further Reading
| - | You can view other papers presented at the Australian Poultry Science Symposium 2011 by clicking here. |
May 2011








