



Efficacy of Infectious Bursal Disease Virus Vaccine Against Various Forms of the Disease
By Yannick Gardin, Vilmos Palya, Luis Sesti and Kristi Moore Dorsey.Introduction
Infectious bursal disease virus (IBDV) is a widespread pathogen causing serious economic losses. Classical virulent and even more very virulent strains caused high morbidity and mortality under field conditions. Recently several strains have been identified, causing subclinical infection when no significant losses are caused by the IBDV itself, but its immunosuppressive effect leads to oftener and more serious secondary infections resulting poor performance in broiler flocks.
A field study was conducted to investigate the situation regarding IBD in several broiler flocks reared in the south of Brazil. Six flocks vaccinated according to regular program, and one flock left unvaccinated, were closely monitored. Decay of maternally derived antibodies, IBDV vaccine take and possible field infections were investigated using serology and virology testing.
Materials and Methods
Selection of farms
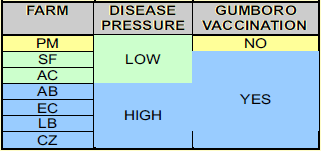
Seven broiler farms located in 3 different geographical areas were selected having different Gumboro disease pressures (see table 1.). Number of animals were around 16 500 17 500 broilers in each flock. Chickens were reared on built up litter, already used for several (3-8) previous flocks.
Vaccination
All farms were vaccinated against Gumboro disease with the exception of farm PM, situated in the “low disease pressure” area (table 1).
All vaccinated flocks were vaccinated once against IBDV, with an Intermediate Plus type Gumboro disease vaccine (Cevac IBD L from Ceva Santé Animale strain Winterfield 2512; 102 EID per dose) administered by drinking water at 14 days of age. The PM farm 50 was left unvaccinated. This farm was previously vaccinated for at least the past 2 years with the same vaccine as indicated above.
Clinical monitoring
All farms were visited weekly. Clinical observations were reported, with special regards to the presence of signs and lesions that could suggest a break of the Gumboro disease.
Samples collection
Serum (15-25/flock/date) and bursa samples (10-10/flock/date) were collected weekly, starting on day 1 up to 42 days of age.
Serological testing
All serum samples were tested for antibodies against IBDV using commercial ELISA kits. All samples were tested with BioChek IBD ELISA kit; maternally derived antibodies were measured at vaccination both with BioCheck IBDV ELISA kit and with Idexx Flockchek IBD-XR ELISA kit. Results were interpreted using Arithmetic Mean Titres (AMT), as well as percentages of positives / negatives sera.
Molecular biological testing
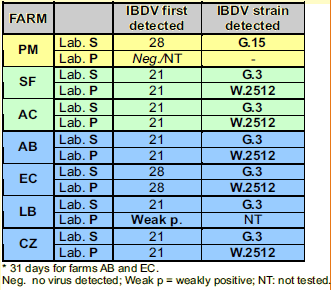
- Laboratory S (Simbios Biotecnologia, Canoas, RS, Brazil). The applied technique has already been published (Ikuta et al.,2001; Majo et al., 2002). According to classification developed by this laboratory, molecular group 3 of IBDVs (G.3) includes the Winterfield 2512 strain (the strain of the vaccine used in this experiment), very virulent Gumboro field strains isolated in Brazil repeatedly fall into the group 11, and strains of Brazilian subclinical variant type isolated in Brazil are classified as members of group 15.
- Laboratory P (Ceva-Phylaxia Viral Research Laboratory, Budapest, Hungary) The RTPCR method used has been described previously (Domanska et al., 2004). RFLP analysis was done by Bsp MI and BSE DI enzymes (for vvIBDV and w2512 strain identification, respectively).
Results
Clinical observations: All flocks, including the PM flock, not vaccinated against IBDV, went through the whole fattening period without showing sign of disease and without showing any clinical sign of the very virulent form of the Gumboro disease.
IBDV serology testing results (figures 1 and 2):
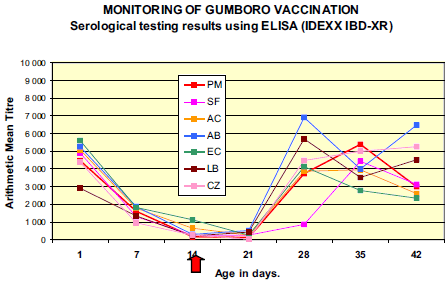
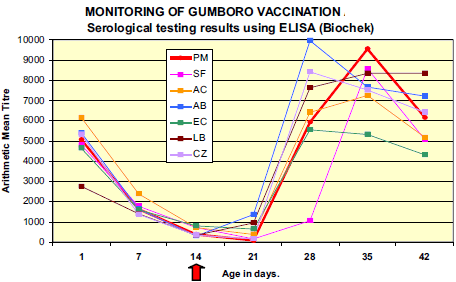
Presence of significant levels of maternally derived antibody (MDA) titres to IBDV was demonstrated in all flocks; with 100 per cent of the samples tested were ELISA positives.
Results from 7 days evidenced decay of MDA in all flocks. On day 21 (one week after vaccination), a clear rise in mean antibody titre value and percentage of positive chicks was observed in flocks LB and AB revealing early immune response to vaccination. Mean ELISA titres of the majority of the flocks peaked at around 28-35 days (AB, CZ, EC at 28 days ; LB and AC at 35 days) with maximum values varying from 5 550 (EC) to 9 974 (AB). Although not vaccinated, flock PM demonstrated serological response to IBDV comparable to other flocks with rise in mean ELISA titre between 21 and 28 days and peak at 35 days (9 536).
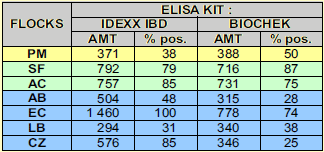
Results obtained with two different ELISA kits at vaccination were comparable (see table 3).
Molecular biology testing
Vaccine take was verified in all vaccinated flocks at 21 or 28 days (see table 2). Field infection of PM farm (non-vaccinated) was confirmed, the strain was identified as a member of G.15 group.
Discussion
IBD Immune status of day-old chicks:
All birds tested on the first day of age (n=117) had rather high level of MDA to IBDV.
Absolute values of AMT were within a range of values 4000 to 6000 (except flock LB; AMT~3000).
Immune status on day 14 and response to IBD vaccination:
Vaccination on day 14 was given in the presence of quite variable antibody status as appreciated by ELISA results (see figure 1 and 2 and table 3). Information given by the 2 ELISAs used for maternally derived antibodies was quite homogenous.
Results of IBDV detection in the bursas using RT-PCR as well as serology results confirmed the take of the IBD vaccine used. One week after vaccination (day 21), almost all bursa samples (5 flocks out of 6) were positive for IBD virus, and sequencing confirmed that the detected IBDV was the vaccine strain (see table 2).
Natural IBDV infection of non-vaccinated PM flock:
Virus detection as well as serology results revealed that non vaccinated PM flock got infected with a field IBDV almost at the same time as the vaccine take was detected in other flocks. Since this virus was not of the very virulent type, no clinical signs were observed but lesions of the bursas were evident. This observation of naturally occurring field infection of flock PM confirmed that vaccination against Gumboro disease corresponds to a real race with the field virus and that precise timing is really critical to achieve optimal protection.
Conclusions
- IBD vaccination using Intermediate Plus IBD vaccine is efficacious in ensuring vaccine virus replication in face of moderately high level of maternal antibody to IBDV.
- Use of commercial IBD ELISA kits allow serological monitoring of successful IBD vaccination or field infection but do not permit to discriminate between the two. Testing results from the two ELISA kits brought similar information although active immunity was detected earlier and stronger by Biochek ELISA.
- There was very good correlation between serology and molecular biology testing results, but only the later technique allowed the determination of the type of IBD virus that infected the chickens.
References
- Domanska, K., Mato, T., Rivallan, G., Smietanka, K., Miinta, Z., de Boisseson, C., Toquin, D., Lomniczi, B., Palya, V., and Eterradossi, N. (2004) Antigenic and genetic diversiti of early European isolates of Infectious bursal disease virus prior to the emergence of the very virulent viruses: early European epidemiology of Infectious bursal disease virus revisited. Arch. Virol. 149:465-480.
- Ikuta, N., El-Attrache, J., Villegas, P., Garcia, E.M., Lunge, V.R., Fonseca, A.S., Oliveira, C., Marques, E.K. (2001). Molecular characterization of Brazilian infectious bursal disease viruses. Avian Dis., 45 (2), 297-306.
- Majo, N., El Attrache, J., Banda, A., Villegas, P., Ramis, A., Pages, A., Ikuta, N. (2002). Molecular characterisation of Spanish Infectious Bursal Disease virus field isolates. Avian Dis., 46, 859 868.









