



Efficacy of Turkey Herpesvirus Vectored Infectious Bursal Disease Virus
A turkey herpesvirus vaccine expressing the VP2 protein of the Delaware variant E strain of infectious bursal disease virus was constructed, write M. Esaki of Zeon Corporation, Tokyo, Japan and Biomune Company, Lenexa, Kansas and K. Moore Dorsey and J. D. Leonard of Biomune Company, Lenexa, Kansas and T. Sato, S. Saitoh, S. Saeki, A. Fujisawa and A. Yasuda of Zeon Corporation, Tokyo, Japan.Summary
This recombinant virus, referred to here as rHVT/IBD, was stable after passages in cell culture and specific pathogen free chickens.
Efficacy of the rHVT/IBD vaccine was evaluated in specific pathogen free chickens and commercial broilers. The rHVT/IBD vaccine was mixed with the SB1 vaccine strain of Marek’s disease virus and administered either in ovo or subcutaneously to day of age chicks. Excellent protection was shown following challenge with either standard or variant strains of infectious bursal disease virus as well as very virulent or very virulent plus strains of Marek’s disease virus. To examine the onset of immunity of the rHVT/IBD+SB1 vaccine, commercial broilers were vaccinated either by the in ovo or the subcutaneous route and challenged at either 1, 2, 3 or 4 weeks old with infectious bursal disease virus. Remarkably, adequate protection was observed through 4 weeks in the vaccinated chickens while maternal antibody level had waned and failed to protect unvaccinated chickens at 4 weeks old. This suggests that the immunity against IBDV conferred by the rHVT/IBD+SB1 vaccine took over as maternal antibody level had declined. Also, long-lasting immunity by the rHVT/IBD vaccine was demonstrated by serological evaluation up to 50 weeks old.
Introduction
infectious bursal disease virus (IBDV), which is highly contagious and may cause one of the two forms of the disease, clinical or sub-clinical disease. Clinical disease of IBD is observed by mortality in chickens older than three weeks of age, while sub-clinical disease is observed in chickens less than three weeks of age via immunosuppression, which is characterized by gangrenous dermatitis, Escherichia coli infections, and vaccination failures. IBDV is a member of the family Birnaviridae based on a genome of double-stranded RNA and in the genus Avibirnavirus. IBDV is divided into 2 serotypes. Serotype 1 consists of viruses causing disease in chickens and serotype 2 strains are not pathogenic. Based on pathogenicity and antigenicity, serotype 1 strains are divided into four subtypes; classical virulent strains, attenuated strains, variant strains, and very virulent strains.
Turkey herpesvirus (HVT) is classified in the family Herpesviridae in the subfamily Alphaherpesvirus. The genus is un-named but is referred to as Marek’s Disease-like viruses that are classified as Marek’s disease virus (MDV) non-oncogenic, serotype 3. Due to the large DNA genome, herpesviruses have been evaluated for use as a viral vector carrying foreign gene(s). For poultry, HVT has been evaluated as a vector for various poultry viral diseases (Morgan et al., Avian Diseases 37, 1032-1040, 1993).
In an effort to produce an IBDV vaccine that protects chicks early in life, we have developed a recombinant vaccine in which the VP2 gene from the Delaware variant E strain of IBDV was inserted into the HVT genome. Efficacy of the rHVT/IBD vaccine was demonstrated in specific pathogen free (SPF) chickens and commercial broilers.
Results
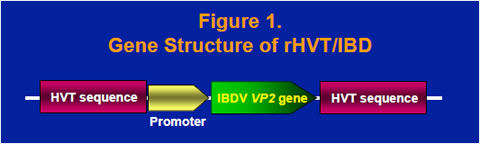
The VP2 gene cDNA from the Delaware variant E strain of IBDV was obtained by reverse transcriptase PCR and cloned into a homology vector with HVT insertion site sequence (Figure 1). A recombinant HVT was generated by co-transfecting HVT genomic DNA and the homology vector into chicken embryo fibroblast cells and purified by expression of the VP2 protein. The recombinant virus, rHVT/IBD, was confirmed to have an expected gene structure and express the 41- kilodalton VP2 precursor protein and the 38-kilodalton mature VP2 protein (data not shown).
Efficacy of the rHVT/IBD vaccine was evaluated. The rHVT/IBD virus was mixed with SB1 (rHVT/IBD+SB1) and administered either in ovo to 18-day-old SPF embryos or subcutaneously to day-of-age SPF chicks. Groups of chickens were challenged with either the USDA standard challenge (STC) strain or the Delaware variant E strain of IBDV at 5 weeks old. After STC challenge, 97% of the in ovo group and 100% of the SQ group were protected. After variant E challenge, 90% of the in ovo group and 93% of the SQ group were protected. All of challenge controls developed bursal gross lesions. Efficacy of the rHVT/IBD+SB1 vaccine against the very virulent RB1B strain of MDV in SPF chickens was also demonstrated (data not shown).
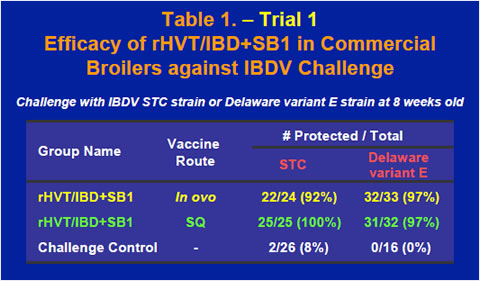
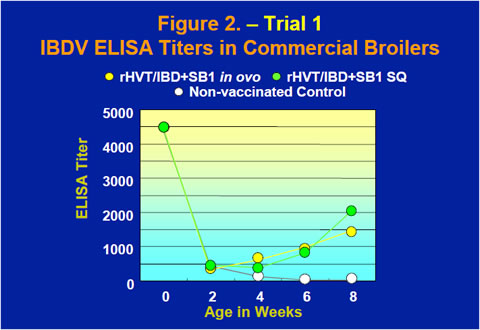
The rHVT/IBD+SB1 vaccine also provided excellent protection in commercial broilers when challenged with either the STC strain or the Delaware variant E strain of IBDV at 8 weeks old (Table 1). IBDV ELISA tests using a commercial IBDV ELISA kit showed that after maternal antibody dropped rapidly through the first few weeks of life, ELISA titers of the vaccinates started to rise between 2 and 4 weeks while the ELISA titer of the non-vaccinated control continued to decline (Figure 2).
Efficacy of the rHVT/IBD-E+SB1 vaccine in commercial broilers was evaluated by co-mingling day-of-age vaccinates with chickens shedding the very virulent plus TKing strain of MDV. The rHVT/IBD+SB1 vaccine provided comparable protection to a commercial HVT+SB1 vaccine (data not shown).
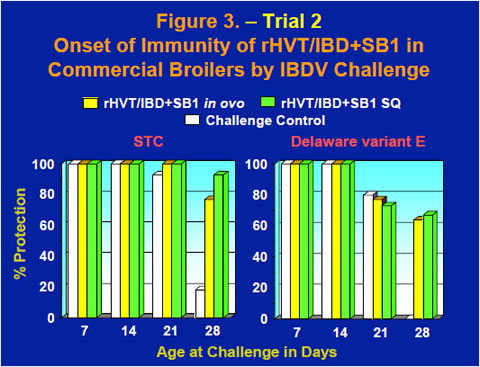
The onset of immunity of the rHVT/IBD+SB1 vaccine in commercial broilers was evaluated by challenging groups of chickens at 1, 2, 3 or 4 weeks old with either the STC strain or the the Delaware variant E strain of IBDV. As maternal antibodies wane, challenge controls started developing gross lesions of IBD at the 3 week challenge and by the 4 week challenge gross lesions were observed in 82% of the STC challenged chickens and 100% of the Delaware variant E challenged chickens (Figure 3). Meanwhile, 64-93% of the vaccinates were protected after challenge at 4 weeks old (Figure 3).
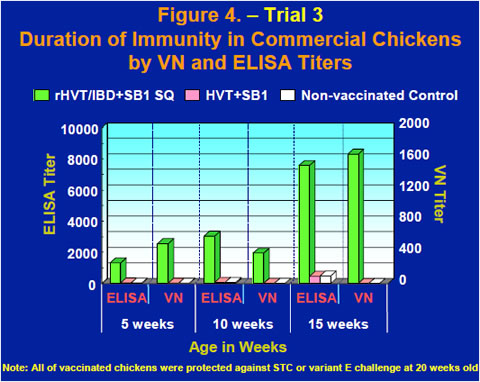
Commercial chickens vaccinated subcutaneously with the rHVT/IBD+SB1 vaccine at one day old were bled at 5, 10 and 15 weeks. Serum was used for virus neutralization (VN) tests using a standard vaccine strain of IBDV and IBDV ELISA tests using a commercial IBDV ELISA kit. High levels of both ELISA and VN titers were observed in chickens vaccinated with the rHVT/IBD+SB1 vaccine, while titers of non-vaccinated controls and chickens vaccinated with commercial HVT+SB1 stayed low between 5 and 15 weeks old (Figure 4).
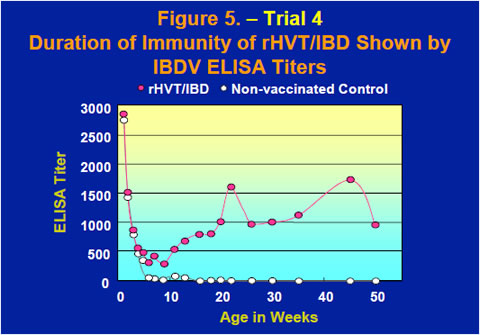
The duration of immunity of the rHVT/IBD vaccine was examined by observing IBDV ELISA titers up to 50 weeks old after inoculation by the subcutaneous route to day-of-age chicks (Figure 5). After maternal antibodies dropped through the first few weeks, the ELISA titers of chickens vaccinated with the rHVT/IBD vaccine came back up to ELISA titers of 1000-1500 and the high level of anti-IBDV antibodies was sustained up to 50 weeks old.
Materials and Methods
Infectious Bursal Disease Virus Challenge. IBDV challenge was conducted by the ocular/intranasal route using 102.7 to 103.6 EID50/dose of the USDA standard challenge strain or the Delaware variant E strain of IBDV. At five or seven days post challenge, all groups were necropsied and examined for bursal gross lesions including peri-bursal edema and/or edema and/or macroscopic hemorrhage and/or discoloration (yellowish) and/or atrophy.
Marek’s Disease Virus Challenge. After challenge with the very virulent RB1B strain or the very virulent plus TKing strain of MDV, chickens were observed until seven or eight weeks of age. The chickens were then necropsied and examined for grossly observable lesions consistent with Marek’s disease including, but not limited to, enlargement of the sciatic nerves or tumors (abnormalities) in the kidneys, spleen, liver, heart, gonad, skin or eyes.
Conclusion
Our results showed that a single vaccination with the rHVT/IBD+SB1 vaccine via the in ovo or the subcutaneous route to day-of-age chicks gave excellent protection against challenge with the STC strain or the Delaware variant E strain of IBDV as well as the very virulent RB1B stain or the very virulent plus TKing strain of MDV and maternal antibodies did not interfere with the protection. A high level of anti- IBDV antibodies elicited by the rHVT/IBD vaccine was sustained at least up to 50 weeks old.









