



Evaluation of Litter Treatments on Salmonella Recovery in Poultry Litter
By J.B. Payne and Susan E. Watkins, Center of Excellence for Poultry Science at the University of Arkansas's Avian Advice - Pathogenic bacterial populations can have a negative effect on the production and health of birds if concentrations are too high. Bacteria cause numerous disease conditions including necrotic enteritis, botulism, gangrenous dermatitis, airsacculitis, and cellulitis.| The Author | |
 Dr. Susan Watkins Extension Poultry Specialist |
|
Introduction
In addition, pathogenic bacterial
populations are also linked to current food safety concerns
at the processing plant. Because of these concerns, USDA
–Food Safety Inspection Service (FSIS) has mandated that
poultry processing plants follow HAACP programs to control
pathogenic bacteria. FSIS is now evaluating the feasibility
of implementing food safety regulations at the farm level.
Should pathogen control begin at the farm level, integrators
and growers will be challenged to reduce pathogen production
during grow-out. Corrier et al. (1999) reported that the
incidence of Salmonella increased in the crop of broilers at
the end of the feed withdrawal period as compared to the
level of Salmonella in the crops at the beginning of the feed
withdrawal period (10% versus 1.9%). The researchers
speculated that the increased incidence of Salmonella was
associated with an increased tendency for the broilers to
consume contaminated litter in the broiler house during
the withdrawal period. Trampel et al. (2000) reported that
Salmonella recovered from carcasses in poultry processing
plants could be due to fecal shedding onto the litter which may
lead to heavy contamination of the bird’s feathers and feet.
Many integrators and growers are currently faced with
disposal problems of used litter. This leads to the re-use of
litter over an extended time frame which could compromise
the poultry producer’s ability to follow proper sanitation
procedures and best management procedures (BMP’s).
Growers then may rely on the use of litter amendments and
disinfectants as their sole source of solving any problems
associated with diseases caused by high bacterial levels.
Unfortunately, in order to cut costs, growers may apply litter
amendments below manufacturer’s recommendations with the
hope of accomplishing somewhat of an improvement from
current conditions of the poultry house.
Litter amendments are commonly used in poultry
houses for the reduction of harmful ammonia levels by
lowering litter pH. It has been shown that by lowering pH
levels, reduction occurs in bacterial concentrations. A study
was conducted to determine if the application of Poultry
Guard at different levels would effectively reduce the
incidence of Salmonella in used litter (Trial 1). A separate
study (Trial 2) was conducted to determine if the application
of Poultry Guard and PLT (Poultry Litter Treatment) would
effectively reduce the incidence of Salmonella as well as
determine at what application rate reduction would occur.
Should a litter treatment be an effective method of
reducing food pathogens in the litter, then the potential for
crop and possibly carcass contamination could be significantly
reduced through the application of a litter treatment prior
to implementing feed withdrawal programs. With reduced
pathogens in the bird’s environment, contamination of the
exterior body should be lowered, thus reducing pathogen
recovery at the processing plant.
Materials and Methods
Bedding material was obtained from one of the University of Arkansas’ commercial broiler houses that serves as a contract production facility for a local poultry integrator. Prior to the experiment, the litter had been exposed to one flock for Trial 1 and three flocks for Trial 2. The original bedding material was kiln dried pine shavings. Litter was placed at a depth of 2 inches in one square foot baking pans. All pans were then covered with aluminum foil and autoclaved for 45 minutes at 121OC to sterilize the litter. Pans were then removed from the autoclave and allowed to cool to room temperature.
Trial 1
Inoculation: All pans were inoculated with 100 ml of
104 CFU/ml nalidixic acid-resistant Salmonella typhimurium
(NAL-SAL). The application rate of 100 ml was chosen due
to its ability to create a good coverage on the litter surface.
Treatments: There were 4 replicate pans of litter per
treatment. The two treatments were top-dressed onto the
litter as recommended by the manufacturer. The four control
pans remained untreated. The treatments consisted of Poultry
Guard at 100 and 150 lb/1000 ft2 application rates. A total of
twelve pans of litter were used.
Sampling techniques: Surface and core samples were
collected from each pan 24 hours after application. Surface
samples were collected using a sterile cellulose sponge
hydrated with sterile skim milk. Core samples measuring
one inch in depth and weighing 25 grams were collected. All
samples were then placed into Butterfield’s Phosphate Diluent
and enumerated onto XLT 4 agar containing nalidixic acid,
which was incubated at 35OC. Litter pH and moisture content
was determined in all groups 24 hours post application.
Trial 2
Inoculation: All pans were inoculated with 50 ml of 105
CFU/ml NAL-SAL.
Treatments: Each treatment was assigned to 16 pans
with 4 application rates of 25, 50, 75, and 100 lbs/1000
ft2. Replicates of 4 were used for each rate along with
4 untreated pans serving as the control. The treatments
consisted of Poultry Guard and PLT. Both treatments were top
dressed onto the litter as recommended by the manufacturer.
Recommended rates were 75-100 lbs/1000 ft2 for Poultry
Guard and 50-100 lbs/1000 ft2 for PLT.
Sampling techniques: Core samples measuring half an
inch in depth and weighing 25 grams were collected 24 hours
post treatment. All samples were then placed into Butterfield’s
phosphate diluent and enumerated onto XLT4 agar containing
nalidixic acid, which was incubated at 35OC for 24 hours.
Litter pH and moisture content was determined in all groups
24 hours post application.
Analysis Results: were analyzed using the GLM
procedure of SAS. All counts were converted to log10 values
prior to analyses. Significantly different means were separated
using the repeated t-test.
Results
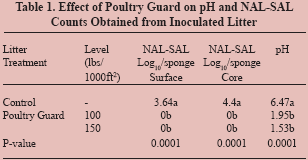
In Trial 1, the application of Poultry Guard at 100 and
150 lb/1000 ft2 resulted in lowering NAL-SAL to undetectable
levels when compared to the control pans. This reduction
was observed in both core and surface samples. Significant
reductions were observed on litter pH, compared to the
control, when both rates were applied (P=0.0001) (Table 1).
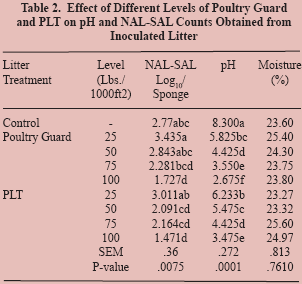
In Trial 2, as compared to the untreated control pans,
both litter amendments resulted in significantly lower levels
of NAL-SAL versus the control when used at the rate of 100
lbs/1000 ft2 (P=0.0075) (Table 2). Also compared to the
control pans, significant differences of NAL-SAL levels were
not observed for either litter amendment when used at rates
of 25, 50, and 75 lbs/1000 ft2. When both treatments were
applied at the 25 lbs/1000 ft2 level, Salmonella recovery was
higher than the control pans. All application rates used for
both treatments significantly lowered pH levels, versus the
control, with the highest application rate having the most
significant effect. Moisture content remained consistent for all
treatments including the control.
Discussion
Litter amendments are often times applied below the
manufacturer’s recommended levels to save costs. When
this practice is used on older litter with high pH levels, lesser
amounts of treatment may only be lowering the litter pH to
ideal levels for bacterial growth. Another consideration is
the possibility of creating litter pathogens somewhat tolerant
to litter treatments by exposing the pathogens to sub lethal
amounts of treatment.
According to Trial 2, rates of 100
lbs/1000 ft2 for the two litter treatments tested are required to
significantly lower levels of NAL-SAL in litter. In Trial 1,
Poultry Guard at application rates of 100 and 150 lbs/1000 ft2
reduce NAL-SAL to undetectable levels, although this was not
observed for the 100 lb. application rate in Trial 2. A possible
explanation for this occurrence could be the difference in
inoculation rates for both trials. Trial 1 received a higher
inoculation rate of 100 ml while Trial 2 received a 50 ml
inoculation rate. The higher inoculation rate would increase
the litter moisture content, possibly causing an increased
activation of the litter amendment. This may explain why we
observed a complete reduction of NAL-SAL in Trial 1. Litter
amendments are not the sole solution for disease problems.
BMP’s and a good sanitation program must be in place in
order to maintain a successful operation. With this in mind,
Salmonella found on carcasses in processing plants could
potentially be reduced with proper sanitation procedures and
the correct use of litter treatments.
References
Corrier, D.E., J.A. Byrd, B.M. Hargis, M.E. Hume,
R.H. Bailey, and L.H. Stanker. 1999. Presence of Salmonella
in the crop and ceca of broiler chickens before and after
preslaughter feed withdrawal. Poultry Sci. 78:45-49.
Trampel, D.W., R.J. Hasiak, L.J. Hoffman, M.C. Debey.
2000. Recovery of Salmonella from water, equipment, and
carcasses in turkey processing plants. J. Appl. Poultry Res.
9:29-34.
Source: Avian Advice - Spring 2005 - Volume 7, Number 2









