



Getting the most out of in-ovo vaccination
Prevention of the main poultry diseases should start in the hatcheryPrevention of the main poultry diseases should start in the hatchery. It is crucial to ensure correct vaccination at hatchery level and to guarantee that good quality and healthy chicks arrive at the farm in order to achieve their full potential. With regard to in-ovo vaccination, it is important to understand its context - the transfer process – to get the most out of it.
The transfer process takes place when eggs are transferred from the setters to the hatchers, and it is a very critical point within the procedures of any hatchery.
Initially, it arose from a need as simple as the impossibility for the chicks to hatch in the incubation trays, but taking advantage of this situation, these devices and processes have been added to it:
- Automation and robots to make the process faster, more ergonomic and with less impact for the embryo.
- Candling devices to remove the unwanted eggs.
- In-ovo devices that allow various vaccines to be applied effectively, with a high level of hygiene and biosecurity and full control of both the dosage and the site of inoculation.
- Systems for the use of infertile eggs or early embryonic deaths, with the possibility for them to be used as by-products.
- Tools to check the status of the incubation or vaccination process at that point.
Focusing on the vaccination process and procedures closely related to it:
Candling
There is no doubt about the importance of removing non-viable eggs during the transfer process. Among other reasons:
- There is a notable increase in biosecurity in the hatchers, where one of the most critical points in this regard occurs, which is the hatching of the chicks.
- Non-viable eggs produce many cold spots in the hatchers, making them more heterogeneous.
- It prevents day-old chicks from being stained as a result of them being broken in the chick take-off system. It also makes the process in this section easier.
- It obtains a performance indicator.
- Option to use non-viable eggs as a by-product.
In-ovo vaccination
In-ovo vaccination consists of delivering vaccines inside the egg with an embryo in the late stage of development, targeting specific sites where the vaccine is capable of stimulating an immune response.
The process of vaccinating eggs during the transfer allows a series of advantages compared to subcutaneous vaccination:
- Reliable and consistent
- Better protection
- High vaccination speed
- Less stress
- Fewer people involved
The vaccines historically applied in-ovo are against Marek, Newcastle, and Gumboro. Recently, a coccidia vaccine has been launched (EVANOVO ®) allowing for a wider range of prevention possibilities in-ovo, and all its advantages.
Regarding the in-ovo devices themselves, the latest improvements consist of:
- Injectors with perforator and needle, which allow the diameter of the latter to be significantly reduced.
- Injector heads that adapt to the shape or angle of each egg within the tray.
- Sensors that guide the injection, allowing the depth of penetration of the needle to be individualized in each puncture, optimizing its application into the amniotic fluid to the maximum.
KPI’s at the transfer
Taking advantage of the process and the fact that eggs are already out of the setters, it is important and appropriate to check both that the incubation process up to this point has happened correctly, and that the in-ovo vaccination has been carried out properly. For this, the most important KPIs at this point in the process are:
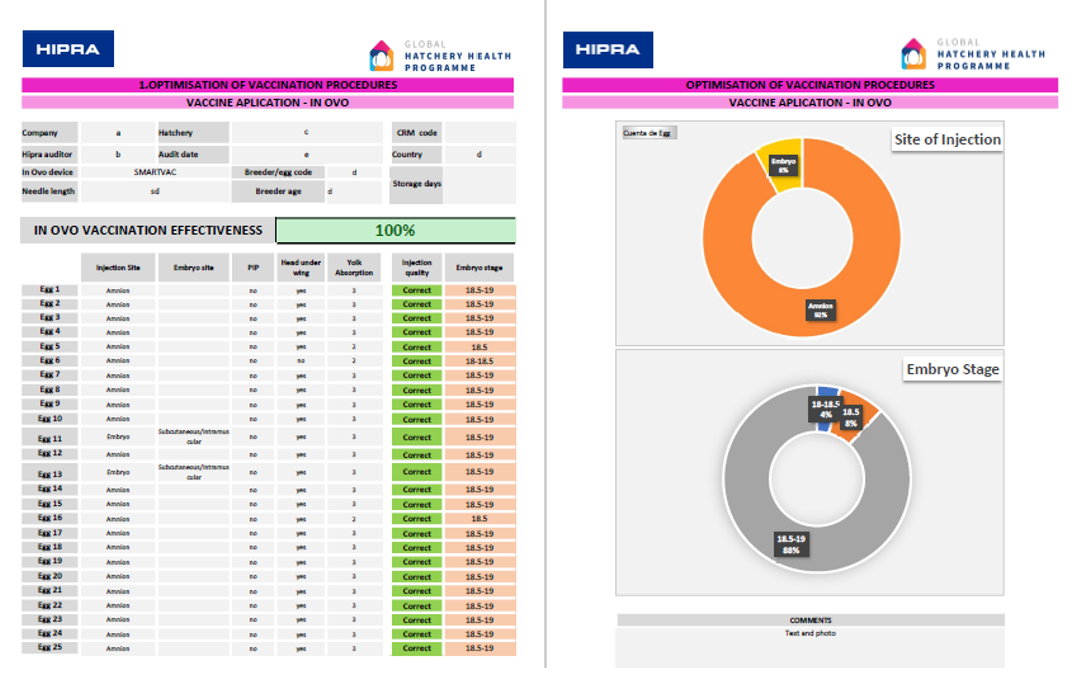
Percentage of viable eggs: The candling provides valuable data to verify whether it is consistent with the curve of its flock, breed, breeder age, storage days, etc.
- Embryo Development: This indicator is of vital importance, since it fulfils a double objective:
- It allows a check to be made as to whether the embryonic development at transfer is as desired, or if it needs adjustments. It also allows the presence of anomalies such as malposition to be verified.
- It ensures that the development stage is within the appropriate range to optimize the application of in-ovo vaccination.
- Site of injection: Consists of determining the point of delivery of the vaccine. The ideal point for any in-ovo vaccine applied is always the amniotic fluid, and subcutaneous application may be correct in many cases, although it is undesirable due to the risk of injury to the embryo.
As in any production process and with respect to data analysis tools, collecting and analyzing data is a must in the new hatchery era.
Tools such as the Global Hatchery Health Programme, developed by Hipra, allow for an exhaustive study of whether both embryonic development and the site of injection deliver the desired results.
Although the hatchery is the heart of the poultry industry, it is just one piece of the puzzle. In fact, it is paramount to have a view of production as a whole interconnected process. This global vision can only be achieved through consistent quality control and data collection. The Global Hatchery Health Programme offers a data science solution that takes into account companies’ business strategy and areas for improvement for both hatcheries and producers in order to support innovative companies in these areas.
With this programme, Hipra once again shows its commitment to disease prevention, by offering innovative and distinctive solutions for the poultry market.









