



In-ovo co-administration of a new vaccine against avian coccidiosis in broilers
Efficacy of EVANOVO® together with GUMBOHATCH® in broilersIn-ovo vaccination in commercial broilers is a growing trend worldwide due to the advance it represents in the automation of the vaccination process. The large production volumes managed by broiler hatcheries and the advantage of individual vaccination and dosage means that this route of administration is ideal under current management conditions. For this reason, more vaccines against diseases for which vaccinations were usually given via other routes of administration are being developed to be administered in-ovo (IO).
On October 2022 HIPRA launched EVANOVO®, the first vaccine attenuated by precociousness against coccidiosis for broilers, specially developed for IO administration. This vaccine represents a clear step forward in the prevention of coccidiosis in broilers thanks to the many advantages the IO route offers. Moreover, EVANOVO® can also be given in the same injection with other vaccines such as GUMBOHATCH®, HIPRA’s next-generation immune-complex vaccine against Gumboro, thereby optimizing and minimizing hatchery vaccination processes.
In-ovo co-administration trial of EVANOVO® and GUMBOHATCH®
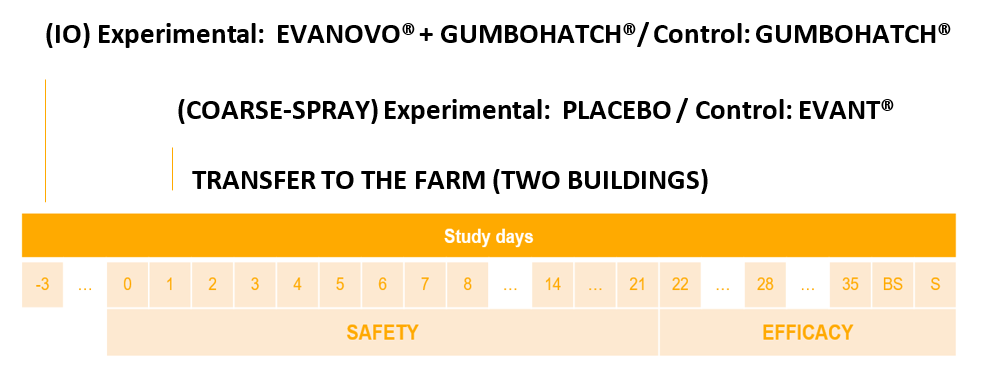
To prove the safety and efficacy of the IO co-administration of EVANOVO® and GUMBOHATCH®, a multicentre, randomized, double-blind and positive controlled field trial was carried out. The IO co-administration of EVANOVO® and GUMBOHATCH® was compared to IO vaccination with GUMBOHATCH® followed by a coarse-spray vaccination with a commercial vaccine against coccidiosis (EVANT®, HIPRA), as a common and effective vaccination approach used on broiler farms.
Design
Three flocks of broiler chicks reared on three commercial farms located in Spain were included in the study. Vaccinations were performed in two different hatcheries after randomization of the eggs into two groups (Experimental and Positive control). In the Experimental group, EVANOVO® was co-administered IO with GUMBOHATCH® to a total of 85,563 eggs at 18 days of embryonic development. In the Positive control group, a total of 85,715 eggs were vaccinated IO with GUMBOHATCH®, at 18 days of embryonic development. After hatching, the Positive control chicks were coarse-spray vaccinated with EVANT® at 1 day of age (Figure 1). Additionally, in one of the hatcheries, co-administration of an in-ovo Marek’s disease vaccine (Rispens) was performed in both groups (Experimental and Positive control). Once on the farms, the two groups were housed in separate rearing units under identical management conditions and monitored up to the end of rearing. Hatching rate, body weight progression, intestinal lesions at different timepoints, feed conversion rate and mortality rate were blindly assessed either as safety or efficacy parameters. Oocyst count profile together with IBD serological response and presence of vaccinal IBD in the bursa were also monitored.
Results
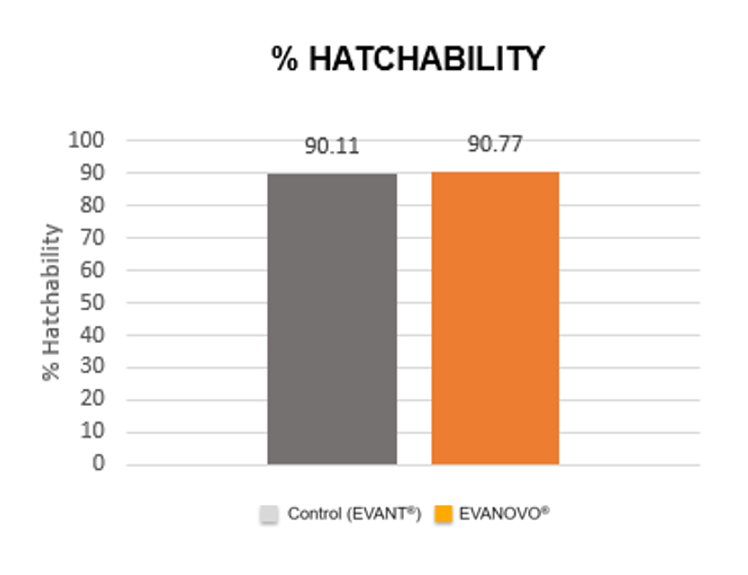
No adverse reactions were reported in any of the groups at hatchery or farm level. In addition, no statistically significant differences were observed between groups, either in the hatching rate (Figure 2) or in the body weight of the chicks after hatching and at 3 weeks of age.
In addition, no clinically relevant intestinal lesions were observed in either of the two groups at the different timepoints evaluated (1 and 3 weeks of age; Figure 3). When evaluating parameters related to IBD, no differences were observed between groups in terms of the incidence and severity of lesions in the bursa of Fabricius due to the vaccinal virus replication, or in the Bursa-Body weight ratio.
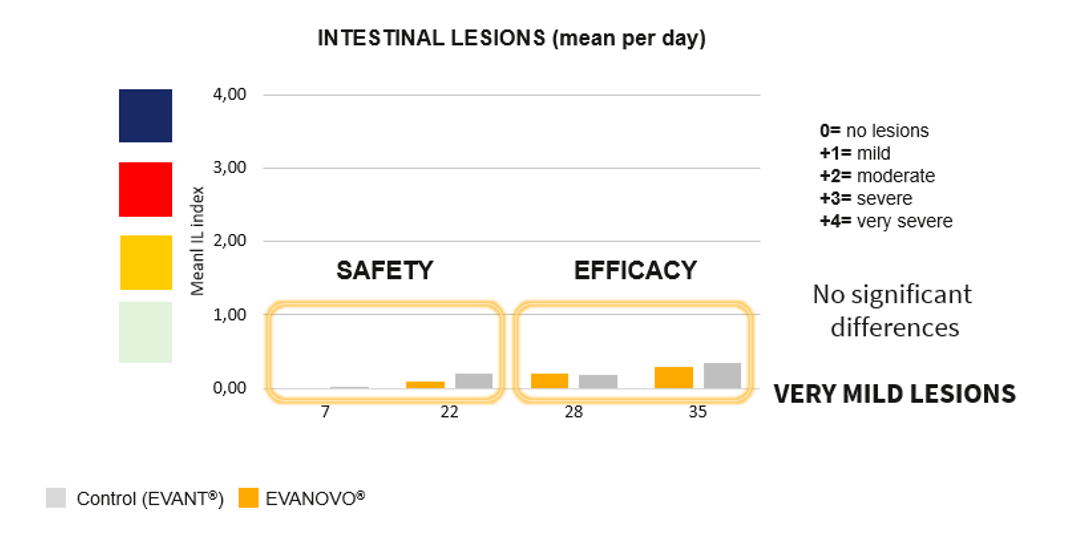
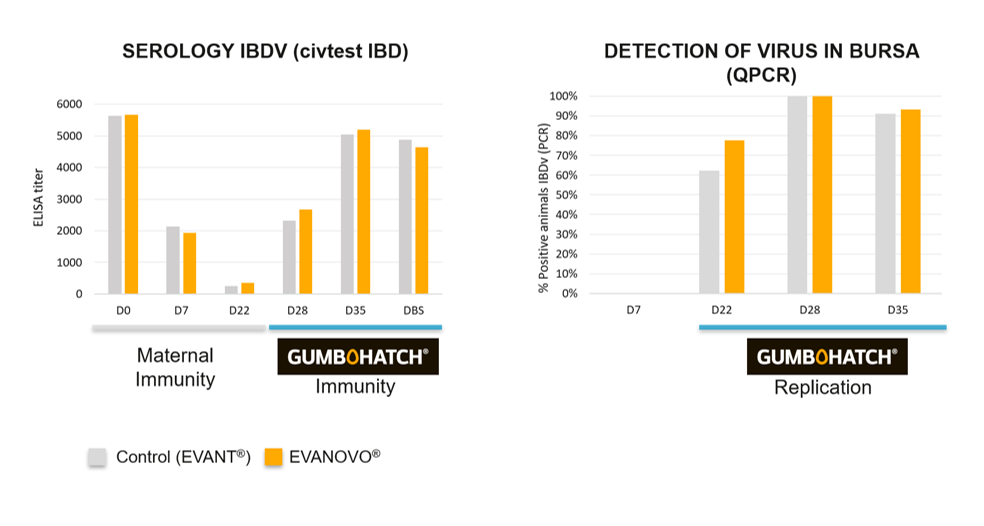
With regard to the efficacy results, no statistically significant differences were observed between groups in the feed conversion rate or in body weight at the end of the rearing period. Nor were any differences observed between groups in the incidence and severity of intestinal lesions at 4 and 5 weeks of age (Figure 3). Finally, no major differences were observed between groups in the oocyst count profile resulting from vaccination against coccidiosis in either the detection of the IBD vaccinal virus in the bursa of Fabricius or in the serological response induced by GUMBOHATCH® (Figure 4).
Conclusions
From the results obtained in this study it can be concluded that the IO co-administration of EVANOVO® and GUMBOHATCH® is a safe and efficacious approach for the immunization of commercial broilers against coccidiosis and IBD.









