



In Search of the Ideal Water Line Cleaner
By Amanda Hancock, Jennifer Hughes and Susan Watkins, Center of Excellence for Poultry Science, University of Arkansas and published in Avian AdviceCleaning poultry drinking water systems can be difficult if systems are dirty or a biofilm slime has become established in the pipes, regulators, and water lines running from the well to the poultry houses. There have been many incidences in which the best daily water sanitation program was less than successful in protecting birds from disease challenges just because the water system was not completely clean before bird placement. The goal of every poultry producer should be to provide birds with the best water supply possible. Unfortunately if growers often use vitamins and other water additives, it is very possible that a biofilm has become established in the pipes and regulators in as little as two to three days.

Biofilms are composed of many types of bacteria and other organisms that live together in a sticky film inside pipes, regulators, and even the nipple drinkers. The biofilm then shields itself by secreting a thick mucous that is not easily penetrated by cleaners such as chlorine or acidifiers such as citric acid. The mucous can even neutralize the cleaner before it has a chance to kill harmful organisms. Then as the biofilm grows and becomes crowded, it releases bacteria into the water and to the birds.
One of the most eye opening cases that drives home the importance of good water sanitation was a turkey barn that had Bordetella positive poults. Bordetella is a bacterial respiratory infection that can set back a flock of turkeys and usually requires antibiotic treatment for successful recovery. The nipple drinker line was cut and a visual inspection of the line indicated no slime. The pipes looked clean. However, when the water regulator was opened, a thick algae growth was present on the pressure seal and the Bordetella was found thriving there (see picture p. 3). That is why if a producer even suspects his water supply or drinkers might be causing health issues in flocks, it is important to pick the right line cleaner and use it at an appropriate rate between flocks when the poultry houses are empty and no birds are present.
The biggest question is: What products give producers the most thorough cleaning for their water systems without damaging the equipment? While many growers have been trained to use products such as citric acid, research results are now showing that when a drinker system is dirty with bacteria, organic acids such as citric acid could be providing the bacteria a food supply and creating a bacterial challenge for new chicks and poults. If the biofilm contains yeast or mold, then lowering the pH of the water with citric acid could actually be creating a more favorable environment for the slime to thrive resulting in clogged drinkers. Most molds prefer a pH of 2 to 5.
Given the fact that many challenges can potentially be present in poultry house water systems, what is the best choice for optimizing line cleaning and eliminating all growth? This was the question that led to the evaluation of several line cleaning products. The objective of this project was to create a microbial rich environment that could potentially shield bacteria and other organisms from cleaners (just like biofilms do) and then determine what products were most effective in reducing or eliminating bacteria, yeast and mold.
Products were evaluated for their ability to kill oxygen loving (or aerobic) bacteria, yeast and mold in the presence of a heavy organic load. These microbes were chosen because they are typically present in contaminated water systems. In an attempt to simulate the slime seen in the Bordetella positive regulator, water containing algae was used for the test. The heavy organic load in this water simulated the challenge for cleaning tough biofilms.
The products tested included a citric acid product; CID 2000®, (20 % stabilized hydrogen peroxide with acetic acid); 35% hydrogen peroxide; Poultry PronTech™ (quaternary ammonium compound); Pro Clean™, (50% stabilized hydrogen peroxide); Proxy Clean™, (50% stabilized hydrogen peroxide); and 6% sodium hypochlorite or house bleach. Table 1 shows the test concentrations for each product.
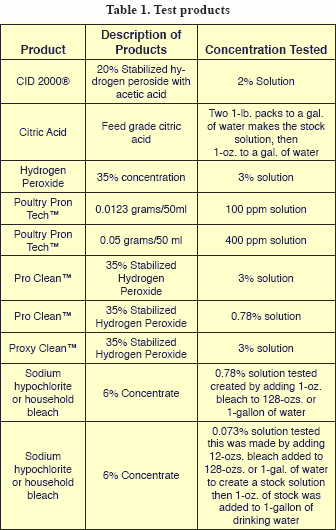
The initial aerobic bacteria counts (APC) ranged from 2 million to 35 million colony forming units per milliliter (CFU/ ml) (Table 2).
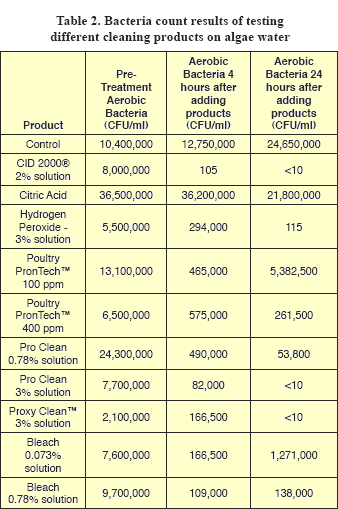
Counts from untreated (control) water increased slightly at both 4 and 24 hours, which showed that conditions favor survival of aerobic bacteria. When the products were compared at four hours post treatment, counts from the CID 2000® hydrogen peroxide treated water had the greatest reduction in bacteria counts with only 105 CFU/ml remaining. At 4 hours post treatment counts from the citric acid treated water showed no reduction. Although all the other products tested reduced bacteria counts, several thousand CFU/ml survived and this level is not acceptable for drinking water systems because it serves as a reservoir of bacteria to re-establish biofilms. At 24 hours, no bacteria were detected in water treated with the CID 2000®, ProClean™ 3%, or ProxyClean™ 3%. The hydrogen peroxide 3% solution also had dramatic reduction in bacteria counts at 24 hours. The bleach solutions tested showed minimal effectiveness in reducing bacterial counts, as did the PronTech™.
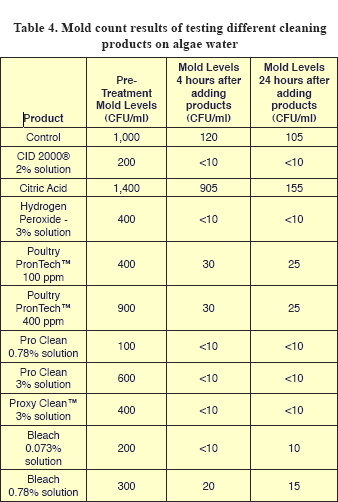
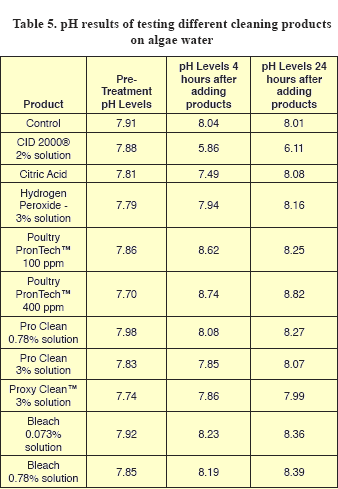
Yeast and mold counts from control samples decreased by about tenfold at 4 hours, but did not further decrease at 24 hours (Tables 3 and 4). Since yeasts and mold prefer to grow in low pH’s (acid conditions), these counts may reflect the fact that pH values for the water used were higher than 7 (alkaline) (Table 5).
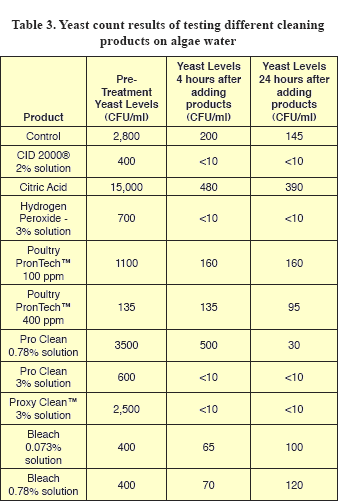
Initial yeast ranged from 135 to 15,000 CFU/ml (Table 3). While all yeast counts except the PronTech™ 400 decreased at 4 hours, levels were undetectable for the 2% CID® 2000, the ProClean™ 3%, ProxyClean™ 3% and hydrogen peroxide at both 4 and 24 hours. Interestingly, yeast counts from bleach treated samples decreased at 4 hours, but increased slightly at 24 hours.
Mold counts from pre-treatment samples ranged from 200 to 1,400 CFU/ml (Table 4). More products showed effectiveness in reducing mold counts than yeast counts (Table 3). Mold counts from CID® 2000, both levels of ProClean™, ProxyClean™, 0.073% bleach solution and 3% hydrogen peroxide decreased to undetectable levels by 4 hours, while counts from citric acid, PronTech™ and 0.78% bleach treated samples decreased less.
The initial pH for the different dirty water solutions was above pH neutral (7) which is not uncommon for many water supplies (Table 5). There were no obvious trends in pH among the treatments. The control showed a slight increase in pH at 4 and 24 hours. The CID 2000® had a drastic reduction in pH at 4 hours, but at 24 hours it increased. The citric acid had an initial lowering of pH, but at 24 hours it increased. Hydrogen peroxide increased the pH at 4 and 24 hours. Both PronTech™ rates had an increase in pH at 4 and 24 hours and this would be expected for ammonia-based products. The Pro Clean at both rates also increased the pH at 4 and 24 hours, as did the Proxy Clean™ and both bleach rates.
Conclusion
The products which showed the most effectiveness in virtually eliminating bacteria, yeast and mold were 2% CID 2000®, 3% ProClean™, 3% ProxyClean™ and 3% hydrogen peroxide (35% concentrate). Citric acid had little impact on the bacteria. The yeast and mold levels tended to become lower no matter what the treatment but were reduced to undetectable levels by the same products that reduced the bacteria. It was also interesting to note that it took up to 24 hours to have the most impact on bacteria levels with the most effective products with the exception of CID 2000®. Knowing that mold typically prefers acidic pH and the samples were slightly alkaline, the environment was not very favorable for mold. The high pH PronTech™ solutions were not very effective but the test was somewhat an unfair test since low concentrations PronTech™ solutions (100 and 400 ppm) were compared to 3% hydrogen peroxide solutions. Future work will focus on stronger concentrations of PronTech™ since it is a high pH product that may have great value in high pH water. Higher concentrations of bleach were not used since strong bleach solutions are known to be damaging to water line equipment.The take home message from this project is water systems which contain a great deal of bacterial growth and slime may very well need products at stronger concentrations to eliminate the challenge. Otherwise, bacteria may remain in concentrations that can return to high levels once the cleaner is removed from the system. Weak citric acid solutions are not good line cleaner choices for dirty systems. To achieve 3% solution concentrations, producers can mix 1.5 gallons of product in 50 gallons of water then use a 1/4th hp submersible pump to add the cleaner at the medicator connection. To determine if water systems might need extra strength cleaning, take apart a regulator. If a coating of slime is present and performance issues have existed in previous flocks that were not management related, then thorough water line cleaning is recommended. A final note, always check with your equipment supplier prior to using any product in your drinker system.

March 2007








