



Manufacturing A Quality Premix
By Avitech - A premix is a mixture of vitamins, trace minerals, medicaments, feed supplements and diluents. It is a value added solution for feeds with sustainable safety and quality. The premix industry is charged with the responsibility of manufacturing a high quality premix consistently, efficiently and economically.
Its main objective is to deliver the micro ingredients in a manner desired by the consumer.
Premixing has progressed from
the simple hand mixing of several
ingredients to mechanical mixing, to
continuous mixing, and now to
computer controlled mixing.
However, the basic concept of
mixing ingredients together to result
in a homogeneous blend has
remained unchanged.
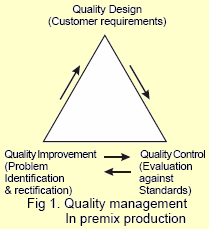 |
A quality premix can be manufactured only through a stringent quality assurance programme and current good manufacturing practices (cGMP).
Quality assurance is a proactive, continuous system for monitoring reproducibility and reliability of a product. It encompasses all the activities undertaken to ensure predetermined standards of a quality premix. Good manufacturing practices covers all the areas of the production process like personnel, facilities, raw materials, quality assurance checks, inventory control, processing, mixing, packaging and delivery.
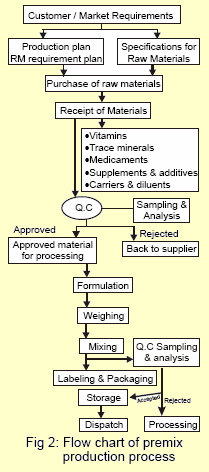 |
Premix manufacturing process comprises:
- Raw Materials
- Selection & Specifications
- Purchase
- Receipt & Storage
- Sampling & Analysis
- Processing
- Formulation
- Weighing
- Mixing
- Packaging
- Labeling
- Storage of Finished Premix
A: RAW MATERIALS
1: Selection & Specifications
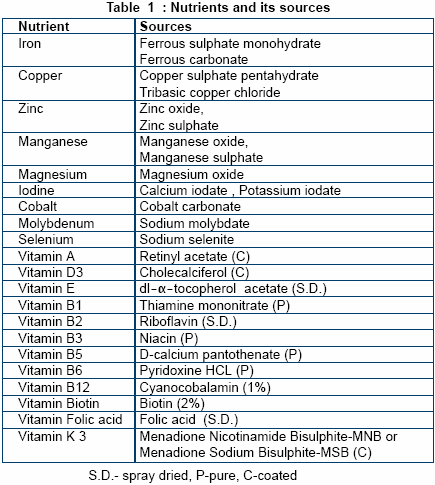
Vitamins and trace minerals are
available in different forms and their
bioavailability varies between
sources. Amongst vitamins the
stability forms an important criteria
whilst bioavailabilty, potency and
reactivity of trace minerals aid in
their selection process. The form of
ingredient selected must also be
easily available, of economic
interest and also impart acceptable
physical attributes to the premix.
In order to maintain the stability
of vitamins throughout their shelf
life it is recommended to procure
more stable derivatives like coated
forms, that are not destroyed even
when mixed with trace minerals.
The spray-dried form of vitamins
improves the flowability of the
premix. The list of nutrients with
their recommended sources is
shown in Table 1.
The specifications for all raw
materials should be based on
recommendations applicable for
particular animal's feed as
mentioned by AAFCO, AOAC,
AFMA, I.P, U.S.P., etc.
2. Purchase of Raw Materials
Raw materials must be
procured from approved vendors
and should conform to the
specifications laid down by the
nutritionist. No material should be
received without a certificate of
analysis.
Purchases should be done
periodically taking care that
sufficient inventory is maintained at
all times. A purchase plan is
desirable in accordance with
production requirement.
3. Receipt & Storage of Raw Materials
The receiver should have
enough information from the quality
assurance program to be able to
recognize the quality of product.
Sacked ingredients should be
checked for identification and
condition. A reference number
should be allotted for each raw
material received into the premises.
The sacked ingredients must then
bear this reference number. Large
consignments are to be weighed at
weigh bridge whereas small ones
by using electronic balance. The
complete details of the raw material
along with its reference number
must be entered into the stock
records.
The raw materials should be
logged in after segregation of drugs
and other nutrients. Extra care must
be taken for labeling. Bags or
containers must be stored in a dry
location on pallets assigned to them
taking care of sufficient space
between the pallets for comfort
loading and unloading.
To prevent
development of stacking resistance
not more than 10 bags are to be
stored on one pallet. Meanwhile
stacked stocks should be rotated to
minimize lump formation, product
degradation, and insect infestation.
The storage area must have
sufficient protection from rodents
and insects. It should be well
ventilated, sanitized and away from
direct sunlight. Depending on the
stability of raw material they must be
stored in environment of controlled
temperature and humidity.
The storage area for approved and
rejected materials must be distinct
in order to prevent any confusion.
4. Sampling & Analysis
Sampling of raw materials is
performed following a quality
assurance programme. To obtain a
representative sample, sampling
should be done from bottom, center
and top layer of the bag using a
sample probe. When large
consignments of raw materials are
received, it is advisable to mix the
raw material in mixer and then
analyze each mixed batch to make
an accurate assessment.
Instruments like H.P.L.C, flame
photometer and spectrophotometer
are used for analysis of raw
materials to obtain accurate results.
Raw materials should be analyzed
using official methods by trained
personnel.
Approved raw materials may be
considered for formulation. If raw
materials are differing in their
particle size but other parameters
are satisfactory then they should be
considered for further processing
like sieving, milling etc. When they
are not meeting the pre-determined
specifications in terms of potency or
purity then they should be rejected.
5. Processing
Processing seeks to modify the physical properties of raw materials to meet the specifications of premix. Processing basically includes:
- Sieving
- Milling
Sieving is a primary process of
removing foreign materials from raw
materials as well as separating
coarse ingredients. The operation
can be carried out in equipments
like vibratory or mechanical sifters.
Care must be taken that the sifter is
cleaned well before and after use to
prevent any sort of contamination.
The 'overs' obtained in the sieving
process may then be ground.
A multimill can be used to
reduce particle size to the desired
screen analysis. Regular checks
should be performed to detect wear
of mill screen and blades.
The sieved and milled material is
then bagged, weighed, labeled and
transferred to the warehouse area
for storage.
B. FORMULATION
 This is an important and critical step
in manufacturing a premix.
Qualified personnel possessing
knowledge and expertise regarding
micro ingredients and powder
technology should formulate a
premix.
This is an important and critical step
in manufacturing a premix.
Qualified personnel possessing
knowledge and expertise regarding
micro ingredients and powder
technology should formulate a
premix.
The formulator has to consider
source of ingredients based on their
physical, chemical characteristics,
bioavailability, their interactions
when mixed, handling
characteristics, and economic
implications on the final product
before any final conclusion is
arrived at.
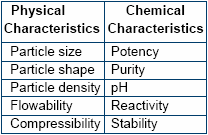
Physical Characteristics
Particles of uniform size,
density and that of spherical shape
blend well to form a homogenous
mixture. The chance of segregation
is thereby minimized. For example
trace minerals available in the
market are invariably found to be
coarse in nature whereas the
vitamins are normally fine powders.
Achieving a homogenous mixing of
these two ingredients would be
difficult (sand and pebble effect).
However, homogenous mixing can
be achieved by processing the trace
minerals to the desired particle size
and improved flowability.
The flow ability of the
ingredients plays a vital role while
handling the powder i.e. before and
after mixing. Poor flowability results
in bridiging, caking and product loss
in the transfer system. Conversely
too fluid a product may cause
flushing.
Chemical Characteristics
Potency of vitamins, trace
minerals and medicaments need to
be considered whilst formulating
high quality premixes. Based on the
customer's requirement the
formulator has to include the
micronutrients considering their
analytical value so that desired
amount can be delivered when
mixed in the feed. No materials
should be incorporated in the
premix without analysis since under
or over addition may have
deleterious effect on the overall
performance of the birds consuming
the feed.
Selection of carrier and its
percentage is important for
formulating a quality premix. It is
generally preferable to leave
sufficient space for a carrier in order
to minimize any sort of interactions
between the active ingredients. The
carrier should serve the functions as
depicted below:
It should neutralize the electrostatic charges present in certain ingredients.
Chemically inert
Primarily have the density, particle shape and particle size compatible with other micro ingredients so as to prevent any demixing in the premix.
Water sequester from other raw materials thereby reducing water activity and improve stability of the premix.
Impart good flowability. Types of carrier widely used in the formulation of premix:
- Organic carriers
- Inorganic carriers
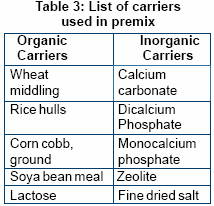 Better premixes can often be
prepared by employing a blend of
predetermined ratio of several
diluents rather than with just one.
The organic carrier absorbs
moisture while inorganic carrier
contributes towards density of
premix.
Better premixes can often be
prepared by employing a blend of
predetermined ratio of several
diluents rather than with just one.
The organic carrier absorbs
moisture while inorganic carrier
contributes towards density of
premix.
The formulator has to consider
all the above - mentioned
parameters whilst preparing the
batch control or manufacturing
record. The batch manufacturing
record serves as a link between the
formulator and actual production.
While preparing the batch sheet the
formulator has to give importance
for the following details:
- Nutrient requirements
- Selection of ingredients
- Potency of ingredient
- Process loss
- Level of free flowing agents
- Level of antioxidant
- Percentage of carrier
- Packing & packaging material
- Inventory of materials
The manufacturing of a premix should follow the batch control sheet under the supervision of trained personnel. The batch sheet should comprise following details:
- Name of the premix
- Code of premix
- Production date
- Batch no. of premix
- Batch size
- List of ingredients to be mixed
- Batch no. of ingredients to be mixed
- Mixing order of ingredients
- Actual quantity of ingredients to be taken
- Mixer name and mixing time
- Instructions regarding packing and mixing
- Provision for signatures
All the above-mentioned details aid in keeping a track of the premix which may be traced in future with respect to customer complaints or product recall. Thus it serves as a control copy.
C. WEIGHING
 Weighing is an important point in
manufacturing of a premix. No
matter how good the formula is, it is
difficult to achieve the desired
nutrient levels in the premix without
precise weighing. Any extra
addition of vitamins may not
improve performance but costs
extra money, whereas lower levels
could depress performance.
Precision in weighing is critical for
certain ingredients like Selenium,
and Roxarsone where mistakes
may even prove toxic.
Weighing is an important point in
manufacturing of a premix. No
matter how good the formula is, it is
difficult to achieve the desired
nutrient levels in the premix without
precise weighing. Any extra
addition of vitamins may not
improve performance but costs
extra money, whereas lower levels
could depress performance.
Precision in weighing is critical for
certain ingredients like Selenium,
and Roxarsone where mistakes
may even prove toxic.
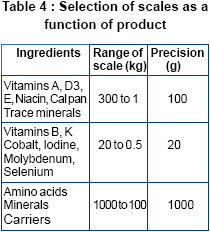
The accuracy of the weighing
balance enables precise weighing.
The accuracy decreases with
increasing size of the scale. As a
general rule, a scale is accurate to
no more than 0.1% of its total
capacity. There is large variation in
doses of different micro ingredients
added in the premix. So weighing
balances need to be sized
according to their use as depicted
left.
Assurance in weighing can be
achieved by calibrating balances
against standard weights. The
balances should be preferably
calibrated daily in the beginning of
weighing and documented
accordingly. They should be
cleaned before and after use and
must be subjected for maintenance
twice a year.
D. MIXING
The mixing process is the heart of any premix-manufacturing unit. In a premix the proportion of ingredients vary considerably; hence in order to obtain a homogenous blend the mixing operation should be divided into two steps, A) Micro mixing and B) Macro mixing
Micro mixing, as the name suggests, is for mixing micro ingredients which weigh less than one percent of mixer capacity. These ingredients should be initially mixed in a smaller capacity mixer like double cone blender. The micro mix so obtained should be then mixed in the large mixer with all other ingredients.
Macro mixing is the actual blending of all components of the premix along with carriers in a batch mixer .The content uniformity of the premix is based on following parameters:
- Type of Mixer
- Mixing Time
- Mixing order
(i) Type of mixer
A mixer selected must be able to
provide homogenous mixtures of
physically adverse particles
incorporated at various levels in the
mix. Horizontal or vertical mixers
can be used. It must meet safety
standards and must be properly
installed. The requirements of a
mixer are:
- Affords good homogeneity with the component included at lowest possible content.
- Short mixing time
- Variable degree of filling, with no loss of mixing efficiency.
- Complete emptying
- Easy Cleaning
- Provision for adding liquids
- Absence of heat during mixing
- Provision to break the lumps
- Less consumption of energy
- Least maintenance cost
- Cost effective
A normal feed mixer is not
recommended for premixes. A
specialized mixer capable of mixing
to a low CV (Coefficient of variation)
is the most desired. Specialized
Nauta mixers are normally capable
of ensuring homogenous mixing.
Examples of mixers: ribbon
mixer, conical screw mixer, mass
mixer, and cylindrical plough mixer
etc.
(ii) Mixing time
The mixing time is also crucial for
obtaining a homogenous premix. It
is evident that shorter mixing time
leads to under mixing while
prolonged mixing time results in
demixing. By trial and error and also
by conducting coefficient of
variation studies, optimum-mixing
time can be arrived for a particular
mixer.
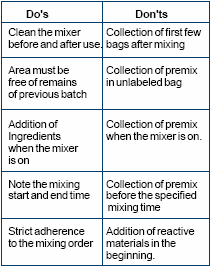 (iii) Mixing order
(iii) Mixing order
The sequence of addition of various
ingredients while loading the mixer
can affect the quality of premix. If
proper mixer loading sequence is
not followed, oil balls, chemical
interactions and particle
segregation can result in a premix.
Regular mixer evaluation should be
accomplished by conducting coefficient
of variation studies. C.V
value of less than 5% is indicative of
excellent mixing. The mean assay
value for the tracer should also be
within the permissible limits of
analytical variation. Any deviation
from the expected result indicates
any one of the following:
- Improper mixing time
- Irregular mixing order
- Selection of bad mixer
- Improper alignment of mixing aids
- Wear out of internal part
- Larger analytical variations
indicative of poor lab analysis Lack of uniformity in a premix is compounded when mixed into finished feed (multiplier effect). Hence utmost care has to be taken whilst manufacturing a premix.
E. PACKAGING
The primary purpose of packaging for premix is to maintain the stability of micronutrients and to protect the integrity of the premix. Improperly packaged premixes experience considerable loss in the potency of various sensitive ingredients. Selection or designing of packaging material should be according to the local climatic conditions. It should bear following properties:
- Provides barrier against light, moisture, oxygen
- No chemical interactions with the premix
- Provides good printing surface
- Sturdy enough to withstand the transport pressure.
The different types of packaging materials that could be use are glass containers, aluminium foil, paper and plastics. Ideally, aluminium foil lined multilayered paper bags provides an excellent barrier against light, moisture, oxygen, odour and flavour. Hence for very sensitive ingredients and where cost is not a constraint, aluminium foil package is the material of choice.
F. LABELING
Labeling of premix serves two purposes:
- Provides complete information about the premix
- Gives an identity to the premix and helps in differentiating from other premixes. The premixes for different segments like layer, broiler, breeder and dairy should bear labels of different colour. This prevents any confusion and mix-ups between the premixes.
- Name of the premix
- Composition
- Dosage of premix
- Net weight of premix contained in the package (in kg)
- Regulatory/Statuatory statements.
- Date of manufacture in month/year
- Date of expiry in month/year
- Batch number
- Storage conditions
- Directions for use
- Name and address of the manufacturer with logo
- Disclaimer note if any
G. STORAGE
The quality of premix is also affected by the storage conditions in the premises until it is transported through distribution channels. The following steps are recommended during warehouse storage:
- The temperature and humidity of warehouse should be controlled below critical levels.
- Keep the area clean, well lit and ventilated with fresh air.
- Store the premix on the pallet meant for it taking care not to store more than 10 bags on each pallet.
- Design the storage areas to facilitate the FIFO (First in First Out) policy, with bags stored in consecutive order so that oldest can be withdrawn first.
- Make separate provision for storing sale return or expired premix
- Keep floors, walls and walkways clean, dry and free of any obstructions.
- Place sinks and bathrooms away from premix storage area.
- Keep the area free from pests and rodents
- No bag should be stored without any label.
When stored under such conditions the consumer is guaranteed of its label claim.
METHODS OF QUALITY CONTROL
- For raw materials
- For production process
- For finished premix
1. The raw materials must be
analysed first and only then
incorporated in the premix once it
meets all the specifications. The
formulator must be strict while
considering the nutrient percentage
in the raw material. Proper
segregation must be done for the
approved and rejected materials.
Care should be exercised while
actually taking the material for
production. The raw material must
be sieved to omit any foreign and
oversized material, if necessary
process before use.
2. The Premix production
process ensures the precise
weighing & inclusion of each
required micro-ingredient in the
premix. This method must be fool
proof and verifiable through a
system of physical (weight) and
book (record) checks.
A preinclusion check should be
performed by quality assurance
personnel whereby random check
is made for the number of bags,
quality of material, their weights,
cleanliness of mixer, integrity of
packaging material and labeling. If
any deviation is observed corrective
steps should be taken immediately
so that the error is not carried on to
the final product.
A post manufacturing check is
necessary to assure that premix
manufactured is free of lumps and
freely flowable.
Cross contamination should be
eliminated while manufacturing
quality premix. It becomes a matter
of concern when drugs get carried
over from one premix to another that
is meant for another segment and
where the contaminated drug is
toxic to other species. There are
three main methods that can reduce
cross contamination in premix:
Flushing- This is a technique whereby an ingredient such as ground grain or any carrier material is run through the system after a batch of medicated premix is produced. This ingredient will pick up much of the contamination in the system and then must be bagged up and used later when premix containing the same medicament is made.
Sequencing-This technique involves the production of feeds containing the same medication at the same time. This reduces the number of times contamination may occur. A run of the medicated premix would then be followed by production of type of premix that would not be affected by a low level of a contamination.
Mixer cleanout procedures-The mixer must be cleaned thoroughly before and after use with brush and compressed air. A thorough washout programme must be performed at least once a week. Care should be taken to ensure that the mixer is clean and dry before use.
3. The quality of finished premix is
assured by analysing the same in
the laboratory .The premix must be
analysed for physico-chemical
properties. The premix should be
dispatched only if all the parameters
are satisfactory. It may be difficult to
analyse each and every batch
manufactured for complete analysis
and hence a sampling plan must be
designed mentioning the analysis
frequency of such premixes.
Premix is a critical input in
feeds. The use of a quality premix is
an important feature in any livestock
operation leading to improved
safety, reliability and performance.
The production of quality premix
thus deserves careful and
professional attention. Product
quality must be built into and not
merely tested in, the product.
Monitoring of all the critical points
affecting the quality of premix is
the best solution for minimizing
the deviations from standards.
It is only through well organized,
adequately staffed
and accurately performed
process and formulation controls
that a desired quality of the premix
may be achieved.
References:
1. P. Raven & G. Walker. Food
and Agricultural Organization
of United Nations, 1980.
2. A. J. Leslie. Quality control in
feed milling, procedures for an
effective program, ASA.
3. D. Monsalve. Key points in
mixing and delivery of micro
ingredients in Feed
Formulations.
4. Micro ingredient Premixing,
Keeping Current. BASF
Corporation.
5. Quality assurance as applied
to micronutrient fortification.
Guidelines for technicians,
supervisors and workers
concerned with Nutition. Eds P.
Nestel, R. Nalubola, and E.
Mayfield.(ILSI)
6. D. Armstrong and K. Behnke.
Premixing MF-2056, Dept. of
Grain Science and Industry.
Source: Avitech Health PVT. LTD. - January 2006









