Mareks Disease: Vaccine handling
By Canadian Poultry Consultants Ltd and Teresa N. Cereno, DVM, Dip. Path., ACPV, Manager of Veterinary Services, AGE, Merial Canada, Inc. - Marek’s Disease (MD) is still considered as one of the most economically devastating poultry diseases worldwide. Billions of dollars are lost in clinical cases as well as in the form of condemned carcasses in the slaughter plants.
 The discovery and eventual application of current vaccines for MD have greatly reduced the occurrence of the “typical” MD in the field. Nowadays, the situation with MD is favorable. However, there are still great concerns that the virus continues to change to a possibly more virulent status which these current vaccines may not be able to protect against. These concerns require vigilance in their study as well as the possibility of developing newer vaccines that can be administered in heavily challenged areas.
The discovery and eventual application of current vaccines for MD have greatly reduced the occurrence of the “typical” MD in the field. Nowadays, the situation with MD is favorable. However, there are still great concerns that the virus continues to change to a possibly more virulent status which these current vaccines may not be able to protect against. These concerns require vigilance in their study as well as the possibility of developing newer vaccines that can be administered in heavily challenged areas.
Almost all poultry operations include MD vaccination for day old birds. Interestingly, doses are sometimes cut or reduced for economic reasons. Some producers giving these fractional doses still claim that this is helping them prevent the disease in the field! We have to remember that some diseases have evolved to a more serious status and started manifesting differently due to abuse and/or misuse of vaccines. The dilution of vaccines should be avoided as manufacturers developed these products to be effective at full dose and any dilution may result in decreased protection.
It is very imperative to know that the vaccine has to be handled properly to make sure that the dose we intend to deliver is what gets into the birds. Most MD vaccines are frozen and cell-associated making them very fragile. A diluted vaccine will not last for a very long time especially after environmental, handling and other adverse conditions are taken into consideration. Use the vaccine within at least 1 hour to avoid loss of titers. MD vaccine can be given in the hatchery either in-ovo at day 18 of the chicken embryo incubation or at day of age via subcutaneous (SC) injection. Either way, it is extremely important to have fully trained personnel to handle, mix and administer the vaccine. These people should always be aware that any mistake in the task can have serious effects. Lastly, choose the right vaccine for your operation. Consult with your poultry veterinarian for the best product to use for the kind of operation that you have.
Here are guidelines that hatchery personnel can use as a checklist to ensure that maximum MD titer is delivered to each and every bird:
A. In-ovo MD vaccination:
1. Vaccine preparation room
-
It is recommended to have a separate MD vaccine room from the rest of the hatchery areas. This should be kept clean with minimum traffic.
-
If using other vaccines in the hatchery, i.e. Infectious Bronchitis, Newcastle Disease vaccine etc., it is recommended to have a separate room for this. Otherwise, label the nitrogen tank properly. There had been cases when these vaccines were given in-ovo because of confusion on the part of the mixer.
-
Have the liquid nitrogen safety sheet displayed prominently near the tank and follow directions.
-
Check the level of liquid nitrogen on a regular basis, if this is not done by your vaccine manufacturer delivery person. The level should not be less than 4 inches.
-
Have the Embrex (or any other in-ovo machine delivery system) recommendations displayed on the wall.
-
Keep working tables cleaned and disinfected with alcohol in between vaccine mixing.
-
Determine the amount of diluent to be used based on the volume of hatch or number of eggs to be vaccinated.
-
The diluent bags must be stored at room temperature. Check that the solution is clear and the bag is not torn. Any cloudiness or abnormal-looking solution should not be used.
-
Record the serial number of the vaccine used.
-
On the diluent bag, record the time the vaccine was mixed
-
Additives such as other vaccines, dye and/or antibiotics must be mixed to the diluent prior to adding the MD vaccine. There should be enough time for these additives to stabilize and reduce possible negative effects to the MD virus.
Remember that antibiotics have the potential to decrease the vaccine titers. Any use of such should be under the supervision of a poultry veterinarian who should have made a prior determination as to the advantage of its application to the birds vis-à-vis a possible negative effect on the MD vaccine.
-
Determine the amount of MD vaccine to be used based on your veterinarian’s prescription.
-
Use separate syringes (5 ml.) and needles (gauge 18) for vaccines and any other additives.
-
Use distilled water in the thaw bath and change this everyday (or every vaccination day).
-
Maintain a constant temperature in the bath (around 80 degrees Fahrenheit).
-
Thaw the number of ampoules needed for one bag of diluent only. Swirl the ampoules very carefully. Vigorous shaking of the ampoule will harm the virus.
-
Ensure that vaccine is thawed completely. Any unthawed particles will not be mixed with the diluent properly. Experience has shown that about 90 seconds of thawing is sufficient.
-
Dry hands and ampoules before opening.
-
Never try to re-freeze vaccines that have been thawed. Discard them properly.
-
If ampoules burst during thawing, the bath temperature maybe too hot.
-
Always treat used ampoules, needles and syringes as biohazard materials and therefore should be discarded according to your region’s regulations.
-
Wash and clean hands before every vaccine preparation and mixing.
-
Swab diluent port with alcohol.
-
Withdraw and inject vaccine slowly.
-
Ampoules and tips should be rinsed with diluent to ensure that all the vaccine PFU’s (plaque forming units) have been transferred from ampoule to diluent bag.
-
Mix the vaccine bag carefully so as not to rupture cells.
-
Transport the vaccine bag into the vaccination room in a cooler with ice pack or ice.
-
Periodically, check the vaccine mixture for any microbial contamination by taking an aliquot sample and plating it onto general purpose bacterial growth medium like Tryptic Soy Agar.
-
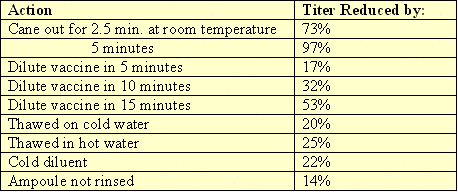
Many years ago, Dr. Halvorson did a study on the effects of handling and mixing errors to the MD titers. The following table reflects his findings. Since that time, some things may have improved as to manufacturing and diluent integrity. The fact remains though that the MD virus is fragile and has to be handled properly in order to obtain the utmost protection.
4. In-ovo (Embrex) machine
-
Ensure that vaccine is used up within 1 hour. Remix the bag periodically during vaccination to prevent the cells from settling down.
-
Embrex provides an insulated bag carrier with ice. Check that the bag is properly cooled.
-
After performing quality checks on the machine, do a test run to make certain that the deficiency has been corrected properly.
B. Day of age SC vaccination:
Follow checklist for the vaccine preparation room, vaccine preparation and vaccine mixing procedure.
Marek’s vaccinator machine (Accuvac, Autovac, Bio-jector):
-
Clean machine every day after use. Do maintenance procedure on a regular basis. Check for leaks and kinked needles. Needles should be changed periodically as a dull and damaged needle can hurt the chicks.
-
If possible, have the shortest tube from the hanging vaccine bag to the machine. A long tube can contribute to the damage of the vaccine PFU’s.
-
Inspect the chicks that have been vaccinated. Look for bleeding that may indicate wrong vaccination site. Some people that are in a hurry vaccinate birds on the head, wings or base of neck.
-
If using dye for monitoring, chicks that are missed will have a visible dye on their heads, on any part of their bodies or on the chick boxes.
-
Watch the vaccinators if they are pulling chicks too fast.
Immunity for MD takes time to develop following vaccination and vaccination actually does not prevent infection. It only prevents the clinical signs associated with it.
Immunity Following Vaccination (Halvorson, 1980)
- Day 1 - 30%
- Day 7-14 - 70-85%
- Day 21-28 - 90-95%
- Severe exposure in the first week - 15-30% maybe susceptible
“The virus wins by mutating and because man abdicates responsibility. The chicken wins because man invests the funds needed to produce better vaccines and to provide barriers to limit the evolutionary drift of the virus”. (Witter, 2001).
Acknowledgements:
Teresa N. Cereno, DVM, Dip. Path., ACPV
Manager of Veterinary Services, AGE, Merial Canada, Inc.
Source: Canadian Poultry Consultants Ltd - June 2004