Mastering frozen vaccine integrity and operator safety from the warehouse to the hatchery
Nowadays, most poultry vaccines are applied in the hatchery. Many of them, such as Marek’s Vaccine or Vector HVT vaccines, are kept in Liquid Nitrogen (-196°C; -320°F) in order to maintain integrity.The process to support effective transport and storage of frozen vaccines is challenging. A flawless cold chain protocol which involves all materials, equipment and procedures must be in place to ensure that the vial is frozen from the time of manufacture until the vaccine is administered.
To do so, first, the frozen vaccines are validated by Quality Assurance (QA) and Quality Control (QC) in the production facilities, before being shipped internationally. They are then controlled upon reception in the local warehouses, as well as during storage, and at time of preparation for delivery to the hatchery.

Liquid nitrogen is required to maintain the live cells in the vaccine. Liquid nitrogen is safe to handle if done so with protective equipment, in adapted facilities and after receiving specific training. Good practices applied at each stage of the distribution flow will guarantee the safety of all people involved in the frozen vaccine logistics.
Hatchery as the center of vaccination regimen
Hatcheries are the first stage of the life cycle of a company. The vaccination program is now mainly applied in hatchery, making it a key milestone of the food chain.
A breakage into the vaccine cold chain in the hatchery can have severe impacts on the whole production chain. The vaccination of a flock with a defrost vaccine will result in a non-vaccine take.
At the farm level, it would affect the production by the potential usage of antibiotic treatment, compromised zootechnical performances, and higher risk of mortality and morbidity. In addition, at slaughter, a low vaccine take may increase condemnations and result in poor carcass quality.
As there are several points to control to guarantee the integrity of frozen vaccines, the process also requires expertise and experience. A non-trained staff may put the integrity of the vaccine at risk in the hatchery. Without training and adapted equipment, the hatchery staff is exposed to a risk of injuries, leading to sequelae.
Therefore, the main challenge for the hatchery is to get the proper training to guarantee both the safety of their team members and the application of a well-preserved vaccine to their flocks.
As with their other innovations Ceva has brought to the poultry market, we would like to help our customers to tackle those critical points at the hatchery.
It is well known that most of our customers feel alone at this stage. Therefore, Ceva has developed another reference service, LINILOG, to complete our value proposition.
LINILOG: Delivering an end-to-end service solution
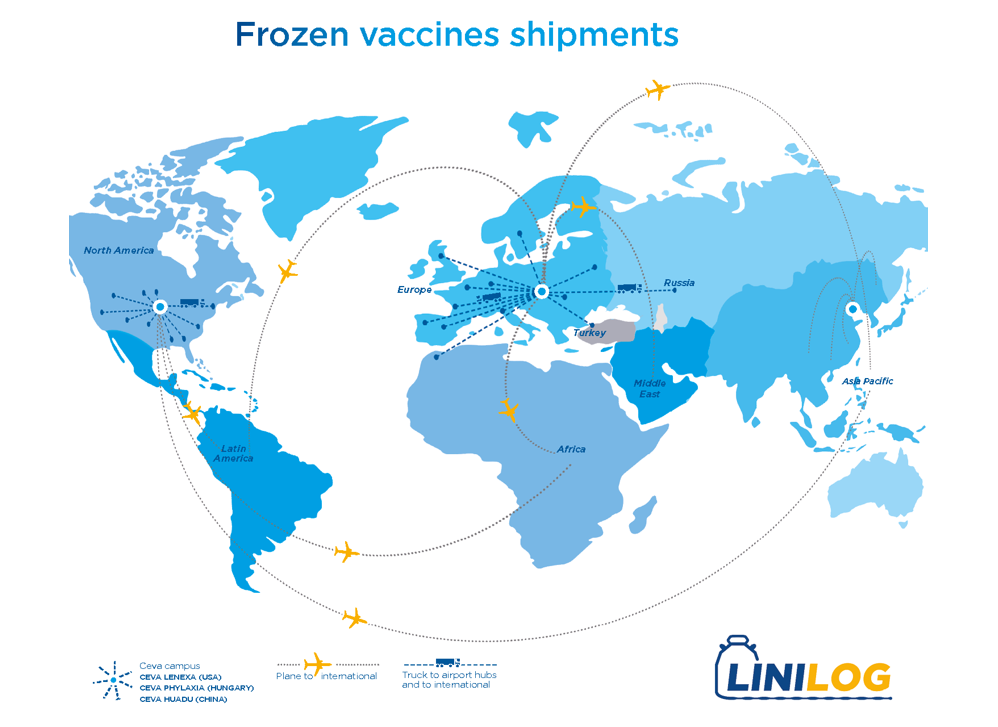
The LINILOG (Liquid Nitrogen Logistics) service was created in 2010 and started in our production plant in the USA, Ceva Lenexa. Since then, it has been deployed worldwide. This service is now in place in our 3 frozen vaccine production plants, and in all our affiliates and partners’ warehouses. The service is also available for the hatcheries receiving frozen vaccines from Ceva and has successfully been implemented in a number of them globally.

Service based on 4 Key Pillars
The LINILOG service contributes to preserving the vaccine integrity and the safety of the people involved, based on 4 pillars:
- CARE OF OPERATOR SAFETY when handling frozen vaccines, thanks to implementing and controlling the application of safety procedures in the warehouse and the hatchery.
- CONTINUOUS CONTROL OF THE VACCINE INTEGRITY thanks to the implementation and the monitoring of the application of preservation procedures in the warehouse and the hatchery.
- VACCINE TRACEABILITY thanks to DEWAR FOLLOW UP from the warehouse, to the delivery to a customer location.
- DELIVERY OF TRAINING AND QUALIFICATION of the teams involved in frozen vaccines logistics.
1. Specific handling conditions are required to protect the health of those involved
Any person involved in frozen vaccine handling should be trained on the emergency first aid procedures and safe practices.
To prevent frostburns, Personal Protective Equipment (PPE) must be provided to personnel and be worn at all times when handling liquid nitrogen. Sets of Personal Protective Equipment are made available for Ceva affiliates and their customers. In 2020, approximately 250 sets were sent to 43 different countries.
Asphyxiation can develop if the room is not properly ventilated. The room must be fitted with an exhaust fan to maintain a safe oxygen level for humans. The usage of an oxygen sensor is advised.
2. Preserving the vaccine integrity
Any exposure to room temperature is minimized, and the vaccine storage conditions are controlled regularly in the production plant, in the warehouse, and in the hatchery.
Special attention is paid to the vaccine in the warehouse when it has to be taken out of the liquid nitrogen upon reception, during transfer from a container to another, in stock control, and in order preparation.
In the hatchery, customers are trained and encouraged to pay special attention to reception and stock control, and vaccine preparation.
After each of these actions, the level of liquid nitrogen and the conformity of the upside down ampoules are monitored and recorded.
The Dewars receive exceptional care at all stages thanks to constant visual control, testing for viability, and digital tracking.
3. Tracing the vaccine means being able to trace all of its storage containers
Using a digital tracking system, Dewars within Ceva’s fleet are identified and located at all times, globally. An obsolete container represents risks of failure or breakage that might not be visible, putting the integrity of the vaccine at stake. Thanks to this tracking system, Ceva ensures that all Dewars in use are viable and can supply new containers to hatcheries when needed.
Together with a complete recording of any monitoring action performed on the Dewars, we can trace the storage life of the frozen vaccines.
4. It has been demonstrated that proper training is required to master the previously mentioned pillars.
Within Ceva, all employees involved in liquid nitrogen logistics, from the supply chain manager to the hatchery specialists and the warehouse team, are trained and qualified in the LINILOG good practices.
All our warehouse and distributor partners go through the same process and are regularly audited using our standards.
Customer hatcheries are provided with Personal Protective Equipment and receive training and recommendations on the storage area when starting to use frozen vaccines. Ceva Vaccination Services & Equipment teams are also available to guide our customers and monitor their liquid nitrogen handling techniques during their regular visits.
Ensuring operator safety in more than 70 countries
After more than 10 years of constant improvement of these practices and their implementation in more than 70 countries, a survey was conducted in 2020 to evaluate the effectiveness of the LINILOG service. It measured the number of injuries due to liquid nitrogen handling and the number of claims registered at the reception of the vaccine at the local warehouse or at the hatcheries due to the defrost vaccine.
In 31 countries, between 2015 and 2019, only 1 injury due to liquid nitrogen handling was declared, and an average of 0.3% of the shipment to the hatchery was facing claims for defrost vaccine suspicion. This proves that the LINILOG service implementation at all stages of the chain helps to master liquid nitrogen logistics. But most importantly, for every claim, a solution was found and an action plan was implemented to prevent the issue from arising again, showing the commitment of the teams to answer the customer's needs.

During their regular hatchery visits, Ceva Vaccination Services & Equipment teams train and audit the hatchery teams on the good practices of liquid nitrogen handling.
In 2019, all the hatcheries visited by Ceva worldwide have shown an average score of 95% of application of the operator safety good practices and 97% of application of the good practices for the preservation of vaccine integrity, proving that regular training is fundamental for good liquid nitrogen logistics.