



New achievements in the development of immune complex vaccines against Gumboro disease. Is there room for improvement?
Gumboro virus immune complex vaccines were developed in the late 1990s with the aim of having a biological product that could be administered in a hatchery and that was capable of providing protection, regardless of the level of maternal antibodies in chicks.However, immune complex vaccines have been shown to be much more than that, as they are vaccines that offer the full protection of live vaccines, but with greater safety and potential for humoral response than the homologous virus alone1. In addition, with immune complex vaccines, it is possible to achieve what is known as "intelligent vaccination" against Gumboro disease, adapting the onset of immunity to the protection needs of each chick, thus avoiding the dreaded gap in immunity which can occur with other types of vaccines when administered to broiler chickens2.
The formulation of immune complex vaccines is based on the combination of a live attenuated vaccine strain with specific antibodies against Gumboro virus. The coating of the virus with the antibody is what provides the virus with protection against the neutralisation of maternal antibodies and gives it its safety properties (less lymphoid depletion, with a faster repopulation of B lymphocytes in the bursa, and prevention of the risk of replication too early in the bursa1,3). The main objective in the formulation of this type of vaccine is, therefore, to provide sufficient protection to the vaccine virus through its total coating with specific antibodies (Figure 1):
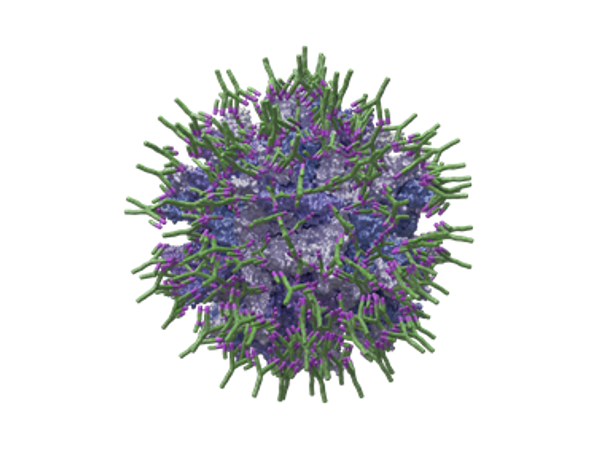
© Source: HIPRA
But how are immune complex vaccines formulated today? Is this coating controlled?
All immune complex vaccines against Gumboro disease are formulated by adding a specific proportion of antibodies, according to the initial titre of the vaccine virus culture (Figure 2). This initial titre is usually determined in the titration substrate, such as embryonated chicken eggs (EID50: 50% infectious dose in egg or embryo) or cell lines (TCID50: dose that infects 50% of the culture tissue) in a similar way to the titration performed with conventional live vaccines. Once the virus-specific antibody has been added, the mixture is subjected to a lyophilisation process, which can induce some loss of titre. Some immune complex vaccines present this virus titre and amount of serum before lyophilisation in their technical specifications, without taking into account the loss of titre that may occur during the process or the correct coating of the virus. In other cases, indirect titrations are carried out after lyophilisation by applying an ELISA test in birds free of specific pathogens (CID50: infectious dose in chicken at 50%), which can take into account the possible loss of titre, but, again, does not guarantee that all virus particles are coated with specific antibodies (the main objective of the formulation of immune complex vaccines).
© Source: HIPRA
Improvements in the formulation of immune complex vaccines against Gumboro disease
A fundamental point to introduce in the formulation of immune complex vaccines is the control of the correct coating of the viral particles. Correct coating of the virus is the only way to ensure that the immune complex vaccine will obtain homogeneous results in the field, since it is this which will prevent the possible loss of vaccine titre when the virus comes into contact with high levels of maternal antibodies.
Two new controls for a new generation immune complex vaccine (GUMBOHATCH®) have recently been developed to achieve this goal:
- Free IgY control: this control determines that a certain amount of free IgY still remains in the final vaccine suspension, which means that all the vaccine particles are necessarily completely coated.
- Neutralisation control: this control involves inoculating the immune complex into embryonated eggs to demonstrate that all vaccine particles have been "neutralised" by their complete coating with antibodies.
However, new tests and additional formulations have also been introduced to make the production process for immune complex vaccines better and more consistent:
- Mixture with fresh virus: immune complex vaccines are formulated by adding a specific proportion of antibodies, according to the initial vaccine culture titre. This initial titration requires a waiting time of 6-7 days between obtaining the culture and the final mixture with antibodies, and, in the meantime, the virus is usually frozen. The new formulation seeks to avoid this waiting time, since it may imply a loss of potency of the virus, formulating the mixture with fresh virus and adding a proportion of antibodies always taking into account the maximum range of the culture titre.
- IgY of egg origin: to date, all immune complex vaccines have used IgY extracted from the serum of hyperimmunised animals as coating antibodies. A new procedure for extracting IgY from eggs has been developed in order to improve the consistency and production capacity of higher quality antibodies (among other advantages).
© Source: HIPRA
- Potency unit test: this test involves the direct titration of the virus in the form of an immune complex after the lyophilisation process, so it is a direct detection of the potency of the lyophilised vaccine. This test replaces the indirect qualification represented by the CID50.
All of these new improvements have served to produce a new generation immune complex vaccine (GUMBOHATCH®) that guarantees the maintenance of the maximum potency of the vaccine and consistent results in the field.
| References | ||||
|---|---|---|---|---|
| 1. Whitfill et al. | ||||
| (1995) | . Avian Diseases | 39, 4, 687-699. | ||
| 2. Gelb et al. | ||||
| (2016) | . Avian Diseases | 60 (3), 603-612. | ||
| 3. Jeuriseen et al. | ||||
| (1998) | . Immunology | 95, 494-500 |









