



Newcastle disease control: The importance of titres in the live vaccine
Introduction
Newcastle disease is a major problem in the poultry industry and is caused by a virulent strain of Avian Paramyxovirus Type I (APMV-I). The Newcastle virus is divided into two classes, class I and class II, class II being the most important for the poultry industry. Class II is divided into XVIII genotypes, however Newcastle disease has just one serotype, the different genotypes being similar antigenically (Afonso, 2017).
Historically, Newcastle disease is a pandemic disease. The first outbreaks occurred in 1926, in Java, Indonesia (Kranvels, 1926) and in Newcastle-upon-Type, England (Doyle, 1927). Today we are in the 4th pandemic wave, caused by the genotype VII group. It was isolated in the mid-1980´s when it started to occur in racing pigeons and affected several bird species, becoming difficult to control due to the lack of controls in racing pigeon production.
According to the OIE (World Organization for Animal Health) terrestrial code, Newcastle disease is an infection caused by a virus of avian paramyxovirus serotype 1 (APMV-1) that meets one of the following criteria for virulence:
a) The virus has an intracerebral pathogenicity index (ICPI) in day-old chicks (Gallus Gallus) of 0.7 or greater or
b) Multiple basic amino acids have been demonstrated in the virus (either directly or by deduction) at the C-terminus of the F2 protein and phenylalanine at residue 117, which is the N-terminus of the F1 protein. The term 'multiple basic amino acids' refers to at least three arginine or lysine residues between residues 113 to 116. Failure to demonstrate the characteristic pattern of amino acid residues as described above would require characterization of the isolated virus by an ICPI test.
In this definition, amino acid residues are numbered from the N-terminus of the amino acid sequence deduced from the nucleotide sequence of the F0 gene, and 113-116 corresponds to residues -4 to -1 from the cleavage site.
The strategy for control of Newcastle disease is based on Biosecurity and Vaccination. The normal vaccination programme consists of a combination of live and inactivated vaccines, the live vaccines often being a Lasota genotype II vaccine.
Why does the choice of Newcastle vaccine have to take the titre into account?
Local protection is important in order to provide early immunity due to the possibility of the chicks having early exposure to a field challenge shortly after vaccination.
The antigen component of CMI (cellular mediated immunity) is the T cell, specifically the T Helper (Th). There are two types of cells, Th1 and Th2. The Th1 is effective in directing the adaptive immune response towards a cell-mediated response. The Th2 cell develops the humoral response. The strain and titres of the live vaccine used will have two effects, one is the CMI and the second effect is the humoral response.
The live vaccines will provide local protection (cellular mediated immunity or CMI) and humoral immunity and will prime the inactivated vaccine, improving its efficacy by increasing its humoral immunity. This is the reason why the ideal vaccination programme will include live (prime and boosters) and inactivated vaccines.
Regarding live vaccines, the level of antibodies is important for control of the clinical signs of the disease, and to reduce shedding of the virus. The minimum live vaccine titre required to control the clinical signs of the disease is 10⁴ EID50(Cornax, I., Miller, P.J. and Afonso, C.L. 2012), however with the minimum titre, the reduction of virus shedding will be limited or non-existent. This is the reason why the usage of higher titres is important.
The HIPRAVIAR®CLON, HIPRAVIAR®CLON/H120, HIPRAVIAR®S and HIPRAVIAR®S/H120 have a minimum titre of 106.5 EID50 for the Newcastle virus; the higher vaccine titres on the market have made a difference in the levels of antibodies, which is especially important because of the mass vaccination processes, especially drinking water and eye drops.
The HIPRAVIAR®CLON and HIPRAVIAR®CLON/H120 are the ideal options because of the lower reaction, more predictable response and higher minimum titre, which make up for possible mistakes in the vaccination process.
A trial shown below was carried out to evaluate the protection induced by two different vaccination plans with HIPRAVIAR® CLON in broilers, against a challenge with a genotype XII strain of NDV isolated in Peru. The efficacy of the 2 vaccination programmes according to the administration route used in the last dose applied was compared.
Materials and methods
120 male 1-day-old broiler chicks from the Ross 308 line were selected, in good health and from the same batch of breeders, free of Mycoplasma Gallisepticum and Mycoplasma Synoviae. At one day of age, the birds in the study were distributed between four experimental groups of 30 animals each:
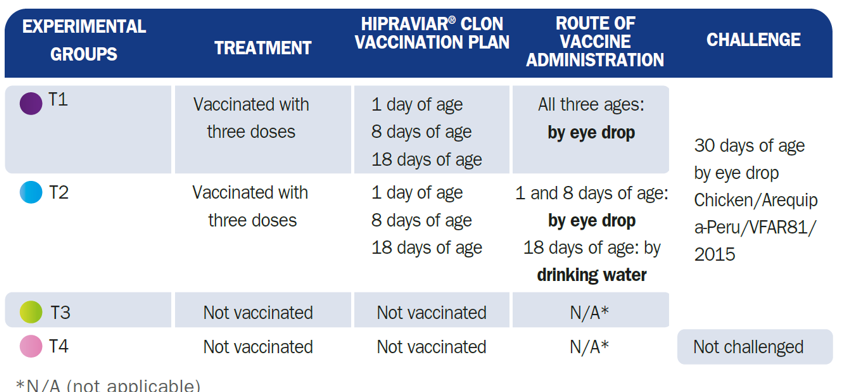
NDV Challenge strain (T1, T2 and T3): 30 days of age
Class II, Genotype XII, sub-genotype XIIa
Chicken/Arequipa-Peru/VFAR81/2015
Intracerebral pathogenicity index (ICPI) - 1.88
Eye drop administration
50 μl / bird - 106/ dose
During the test, clinical signs, mortality and nervous sequelae were evaluated (T1, T2 and T3):
From 2 days after challenge until 10 days after challenge:
All birds were checked
Presence=1
Absence=2
General clinical signs: depression, diarrhoea, helicopter feathers, rhonchus, facial oedema, conjunctivitis, sneezing, nasal discharge and ocular discharge
Nervous signs: complete paralysis, opisthotonus, twisting of the head and neck.
Results
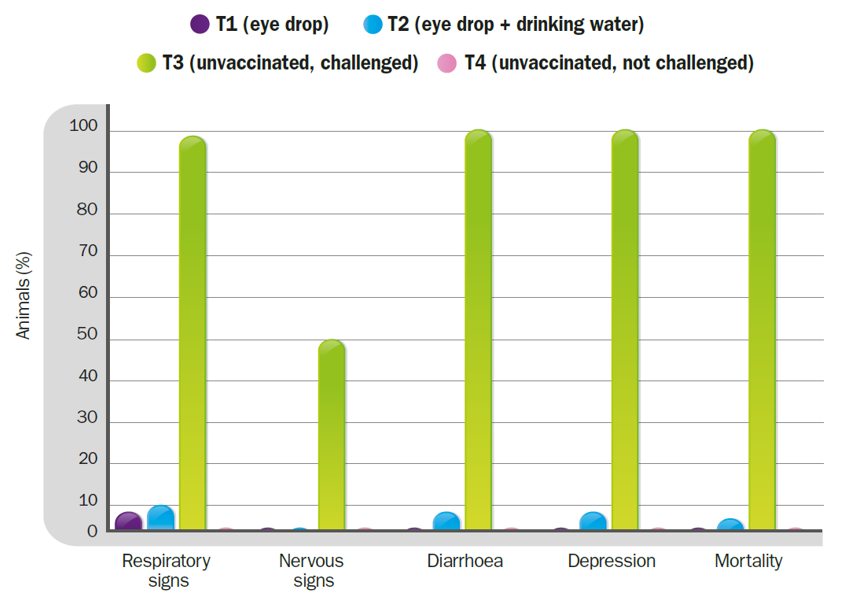
Clinical signs and mortality after the challenge with Chicken/Arequipa-Peru/VFAR81/2015.
Conclusions
HIPRAVIAR® CLON vaccination programmes evaluated in this study demonstrated sufficient efficacy to significantly prevent the mortality and clinical signs induced by the Chicken/Arequipa-Peru/VFAR81/2015 genotype XII velogenic strain of NDV in broilers.
This is a new demonstration of the protective capacity of HIPRAVIAR® CLON against different genotypes of Newcastle virus.
Other authors have shown that vaccines based on strains related to La Sota can reduce mortality and clinical signs caused by different genotypes.
III / IV / V / VI / VII / IX
HIPRAVIAR® CLON, a vaccine virus with respiratory tropism, was able to provide protection against a velogenic viscerotropic NDV.
The change of administration route from ocular to oral, in the last dose of the vaccination plan, decreased both the effectiveness of protection against mortality (T1-0% Vs T2-3%) and the efficacy in preventing the onset of clinical signs (T1-7% Vs T2-10%).
These results could be explained by the fact that ocular administration has a greater antibody response and provides better protection against exposure to NDV compared to the oral route, as other authors have previously observed.
We should not rule out the effect of the method of vaccination.
The trial below shows the importance of the live vaccine titre together with an inactivated vaccine in developing a good level of protection.
Why do live vaccine titres affect the humoral response?
In a recent study conducted in the Laboratory of Avian Pathology of the Faculty of Veterinary Medicine, National University of San Marcos in Peru, 200 healthy bb Hy-Line laying chickens showing antibodies against Newcastle disease and Infectious Bronchitis were used. They were divided into 4 groups of 24 birds each and a control group (T5) of 30 birds which were not vaccinated. This study was performed at the experimental unit of the Pathology laboratory of the Universidad Nacional Mayor de San Marcos
Five groups divided randomly were named T1, T2, T3, T4 and T5. Four groups (T1, T2, T3 and T4) were vaccinated at the same ages: 2, 8 and 11 weeks using live Newcastle vaccines. T1 and T2 were vaccinated using HIPRAVIAR® S/H120 (combination of Newcastle LaSota Strain with a minimum titre of 10⁶∙⁵ and Infectious Bronchitis H120 Strain with a minimum titre of 10³). In the second week, a locally manufactured vaccine (LaSota Strain/ H120) and in the eighth week and eleventh week the HIPRAVIAR® CLON/H120 (combination of Newcastle CL/79 Clon Strain with a minimum titre of 10⁶∙⁵ and Infectious Bronchitis H120 Strain with a minimum titre of 10³). T3 was vaccinated using HIPRAVIAR® CLON/H120 in the second, eighth and eleventh week. T4 was vaccinated using a locally manufactured combination LaSota/ IB vaccine in the second week, eighth week and eleventh week. The complete programme is shown in the table below.
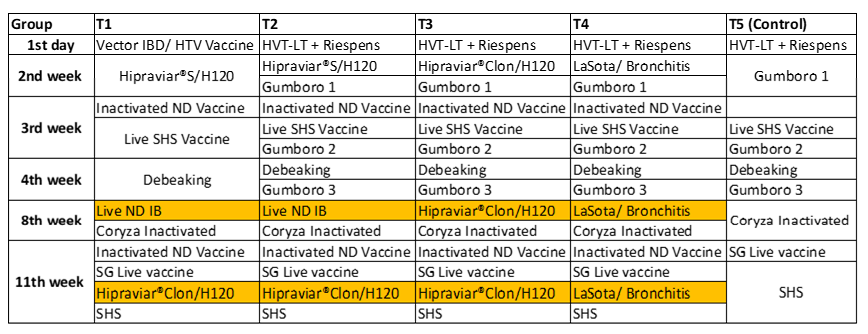
*The inactivated vaccines for Newcastle disease were the same in all four groups.
ELISA
Antibodies against Newcastle disease were detected using the CIVTEST® AVI NDV kit. The CIVTEST® AVI NDV is an indirect enzyme-linked immunoassay. The specific antigen of the Newcastle disease virus (NDV) is coated on 96 well plates. Upon incubation in the test well, antibody specific to the NDV binds to the coated antigen and remains in the well after washing the unbound material, then a conjugate is added that binds to any chicken antibody. After that, unbound conjugate is washed away and enzyme substrate is added. The colour appearing in each well is proportional to the amount of chicken antibody specific to the NDV present in the diluted sample.
Twenty samples per group were taken at 14 weeks of age, the samples were analysed at the Diagnos Hipra Laboratory in Lima, Peru, following all the manufacturer’s procedures. The results for each group were analysed based on the arithmetic titre. The results were statistically compared using ANOVA (Analysis of Variance).
Results
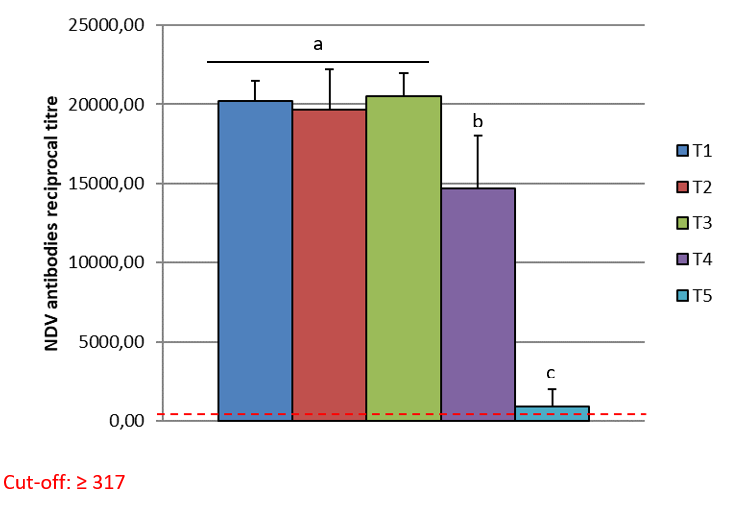
The cut-off for the CIVTEST® AVI NDV is ≥ 317. The serological results are shown in the table below (Table 2).
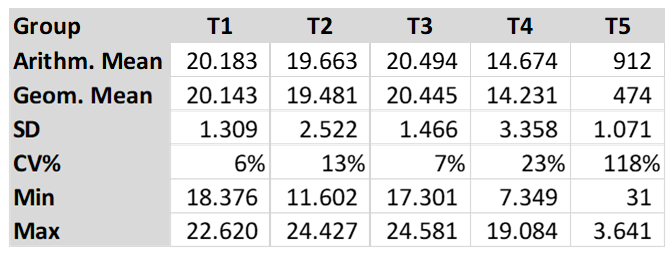
The arithmetic mean titre for T5 (control group) was 911.6. Groups T1, T2 and T3 had the titres shown below, with no statistical difference between the three groups (T1, T2 and T3). As per graphic one, the different letters show statistical difference, a, b and c. Group T4 had an arithmetic mean titre of 14673.85 and was statistically different to the other groups, as per graphic one. The Coefficient of Variation (CV %) in the Groups T1, T2 and T3 was lower than 13% and the (CV%) in T4 was 23% and T5 was 118%. The T5 showed a low titre and high CV.
NDV Titre | NDV Antibody Status |
0-219 | Negative |
219-317 | Suspect |
317 or greater | Positive |
Discussion
The results showed higher antibody levels in T1, T2 and T3 (T1 20,183, T2 19,663 and T3 20,494) compared to T4 (14,674). The Arithmetic Mean Titre did not show any statistical difference between the three groups (T1, T2 and T3) as per Graphic 1. Groups T1 and T2 were vaccinated using HIPRAVIAR® S/H120 vaccine (combination of Newcastle LaSota Strain with a minimum titre of 10⁶∙⁵ and Infectious Bronchitis H120 Strain with a minimum titre of 10³) in the second week vaccination and in the eighth week a local NCD LaSota/H120 vaccine. T3 was vaccinated using HIPRAVIAR® CLON/H120 (combination of Newcastle CL/79 Clon Strain with a minimum titre of 10⁶∙⁵ and Infectious Bronchitis H120 Strain with a minimum titre of 10³) in all live Newcastle vaccinations. Groups T1, T2 and T3 showed lower CV % as per Table 2 compared to T4 and T5. Those three groups were vaccinated using Hipra live vaccines and generic inactivated vaccines at the same ages. T5, as expected for a control non-vaccinated group against Newcastle disease, had lower antibody levels and a higher CV. Group T4 was vaccinated using a locally manufactured LaSota strain and had an arithmetic mean titre of 14673.85 and was statically lower than T1, T2 and T3 and also had a higher CV % of 23%. T1, T2, T3 and T4 had the same locally manufactured inactivated ND vaccine in the third and eleventh week. The live vaccines in the vaccination programme are essential for local immunity but are also important for humoral immunity, Kaczynski et al. (2013). It is important to have high levels of antibodies to protect the flock against the disease and reduce shedding of the virus, Miller et al. (2013).
Conclusions
The results of this study showed a higher level of antibodies with statistical significance in groups T1, T2 and T3 compared to T4. T1, T2 and T3 had a maximum CV % of 13% and T4 had a CV of 23%. As the inactivated vaccines were the same, the live vaccines improved seroconversion, which was higher and with lower CV % in the groups vaccinated with Hipra vaccines. The Arithmetic Mean Titre between Groups T1, T2 and T3 did not show any statistical difference. In group T3, the Newcastle vaccine used was HIPRAVIAR® CLON/H120(CL/79 Clon strain combined with H120) and showed a similar response to T1 and T2 in which the vaccine used was HIPRAVIAR® S/H120 (LaSota strain combined with H120) in the second and eleventh weeks. The results of this trial show the importance of live vaccines in the vaccination programme, not just because of the cellular immunity but also as a primer to the humoral response.
If you are interested in more HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120 trials like the two above, you can access them by clicking on the link below:
https://www.hipra.com/portal/en/hipra/animalhealth/products/detail/hipraviar-clon
| References | ||||
|---|---|---|---|---|
| Tonya L. Taylor, Patti J. Miller, Timothy L. Olivier, Enrique Montiel, Stivalis Cardenas Garcia, Kiril M. Dimitrov, Dawn Williams-Coplin, and Claudio L. Afonso | ||||
| (2017) | Repeated Challenge with Virulent Newcastle Disease Virus Does Not Decrease the Efficacy of Vaccines.. Avian Diseases: | June 2017, Vol. 61, No. 2, pp. 245-249. | ||
| Ingrid Cornax, Patti J. Miller, and, and Claudio L. Afonso | ||||
| (2012) | Characterization of Live LaSota Vaccine Strain–Induced Protection in Chickens upon Early Challenge with a Virulent Newcastle Disease Virus of Heterologous Genotype. Avian. Diseases Digest: | September 2012, Vol. 7, No. 3, pp. e7-e8. | ||
| Orsi MA, Doretto Júnior L, Reischak D, da Silva LHA, Spilki FR, Buzinaro MG, Arns CW | ||||
| . Brazilian Journal of Poultry Science | ||||
| Cornax, I., Miller, P.J. and Afonso, C.L. | ||||
| (2012) | Characterization of Live LaSota Vaccine Strain–Induced Protection in Chickens upon Early Challenge with a Virulent Newcastle Disease Virus of Heterologous Genotype, genotypes III, VIg, VIb, VIId, and IX.. AVIAN DISEASES | 56:464–470. | ||
| Calnek, B.W, Barnes H. J, Beard C. W, Mcdougald L. R, Saif Y. M, | ||||
| (2000) | . Disease of Poultry. Cap 20. | 2nd edtion. 567 pp. | ||
| Hy-Line International. | ||||
| (2018) | Hy-Line variety Brown, commercial management guide.. Hy-Line International. Iowa, United States. | 41 pp. | ||
| Alexander, D. | ||||
| (2003.) | Newcastle Disease and Other Avian Paramixoviridae Infections. In: Diseases of Poultry, Cap 2. 11th Edition. Saif, Barnes, Glisson, Fadly, Mc Dougald, Swayne (eds.).. AAAP Iowa State University - USA. | 63-87. | ||
| ICTV,. International committee on taxonomy of viruses. | ||||
| (2019) | In: Virus Taxonomy: 2018b Release, | |||
| Miller P.J and Koch G., | ||||
| (2013) | Newcastle Disease, Other Avian Paramyxoviruses, and Metapneumovirus Infections. Newcastle disease.. Diseases of Poultry, 13th Edition. Ed. By Swayne, D.E., Glisson, J.R., Mc Dougald, L.R., Nolan, L.K., and D.L. Suarez. Edit. Willey-Blackwell Publishing. Iowa, USA | p. 89-107. | ||
| Umali, D.V., Ito, H., Shirota, K., Ito, T., & Katoh, H. | ||||
| (2015) | Atypical velogenic Newcastle disease in a commercial layer flock in Japan.. Poultry Science | 94 (5), 890-897.doi: 10.3382 / ps / pev011 | ||
| Khorrajiya JH, Pandey S, Ghodasara PD, Joshi BP, Prajapati KS, Hodasara DJ, Mathakiya RA | ||||
| (2015) | Patho-epidemiological study on genotype-XIII Newcastle disease virus infection in commercial vaccinated layer farms.. Veterinary World | 8 (3): 372-381. | ||
| Chumbe, A., R. Izquierdo-Lara, L. Tataje, R. Gonzalez, G. Cribillero, A. E. Gonzalez, M. Fernandez-Diaz, and E. Icochea. | ||||
| (2017) | Pathotyping and Phylogenetic Characterization of Newcastle Disease Viruses Isolated in Perú: Defining Two Novel Subgenotypes Within Genotype XII.. Avian Dis. | 61: 16-24. | ||
| Cornax, I., P. J. Miller, and C. L. Afonso. | ||||
| (2012) | Characterization of live LaSota vaccine strain-induced protection in chickens upon early challenge with a virulent Newcastle disease virus of heterologous genotype.. Avian Dis. | 56: 464-470. | ||
| Liu, X. F., H. Q. Wan, X. X. Ni, Y. T. Wu, and W. B. Liu. | ||||
| (2003) | Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated fromoutbreaks in chicken and goose flocks in some regions of China during 1985-2001.. Arch. Virol. | 148: 1387-1403. | ||
| Jeon, W. J., E. K. Lee, Y. J. Lee, O. M. Jeong, Y. J. Kim, J. H. Kwon, and K. S. Choi. | ||||
| Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain. J.. Vet. Sci. | 9: 295-300. | |||
| Bhuvaneswari, S., K. G. Tirumurugaan, P. Venkatesan, K. P. Manesh, and K. Kumanan. | ||||
| (2017) | Evaluating the efficacy of LaSota vaccination induced protection in chickens upon challenge with a genotype IV strain of Newcastle disease virus.. Virus disease. | 28: 328-336. | ||
| Miller, P. J., C. L. Afonso, A. J. El, K. M. Dorsey, S. C. Courtney, Z. Guo, and D. R. Kapczynski. | ||||
| (2013) | Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses.. Dev. Comp Immunol. | 41: 505-513. | ||
| Al-Garib, S. O., A. L. Gielkens, D. E. Gruys, L. Hartog, and G. Koch. | ||||
| (2003) | Immunoglobulin class distribution of systemic and mucosal antibody responses to Newcastle disease in chickens.. Avian Dis. | 47: 32-40. | ||
| Dimitrov, K. M., C. L. Afonso, Q. Yu, and P. J. Miller. | ||||
| (2016) | Newcastle disease vaccines-A solved problem or a continuous challenge?. Vet. Microbiol. | |||
| Sasipreeyajan, J., Areeraksakul, P., Khanda, S. | ||||
| Protective Efficacy of Live LaSota Strain Newcastle Disease Virus Vaccine in Layer-Type Chickens.. Thai J. Vet Med. | 46 (2): 195-200, 2006. |









