



Poulvac Procerta HVT-ND proved safe and effective when co-administered
Co-administration with IBD immune-complex vaccines, Marek’s strain CVI-988 safe and effectiveWhen faced with the threat of highly virulent viruses and the prevalence of multiple poultry diseases in production systems, a range of vaccination options is often needed.
Previous studies have demonstrated that the recombinant vector vaccine Poulvac® Procerta™ HVT-ND, when administered in ovo or subcutaneously at hatch, can protect birds against Newcastle disease (ND) and Marek’s disease (MD) from 19 days and 5 days of age, respectively, right through the production process.[i]
But with the two diseases and infectious bursal disease (IBD) often appearing simultaneously — and with particularly virulent strains of the causative viral pathogens circulating — demand for simultaneous use of vector vaccines with immune-complex vaccines or MDV CVI vaccines is growing.
In new studies by Zoetis, Poulvac Procerta HVT-ND proved safe and effective when simultaneously applied with Poulvac® Bursaplex® and Poulvac® Magniplex live immune-complex vaccines, as well a Zoetis modified-live frozen vaccine based on the Marek’s disease virus (MDV) CVI-988 strain.
Standardized testing protocols
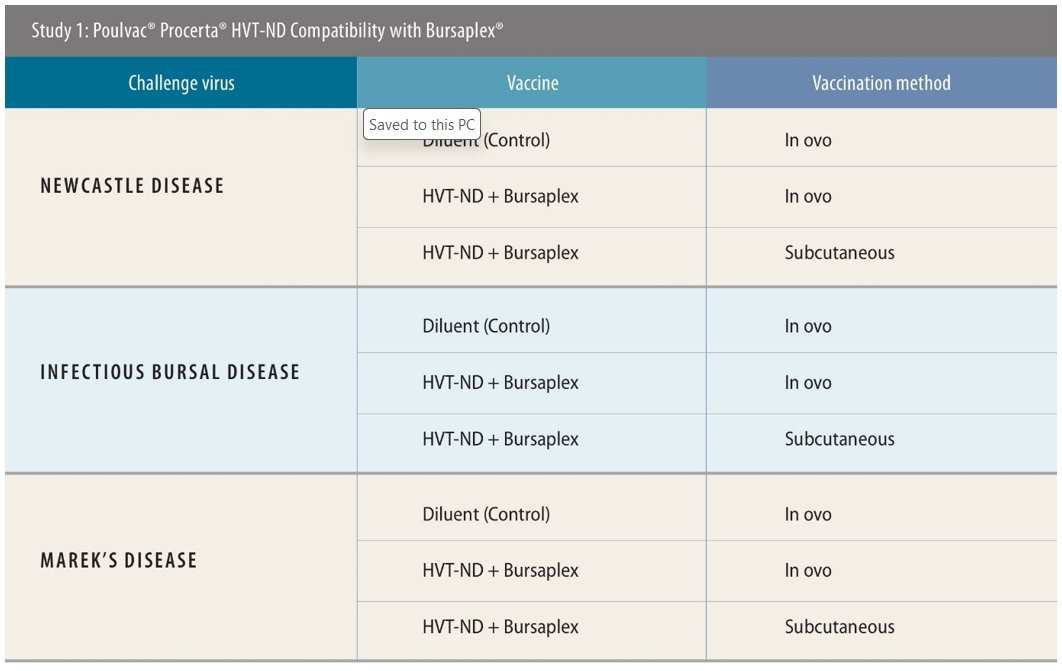
The first two studies were designed to assess the performance of the Bursaplex and Magniplex vaccines, respectively, when used alongside Procerta HVT-ND.
Experimental designs were similar across both these studies. All used a negative control of diluent only. Birds were challenged 28 days post-vaccination with ND, 34 days post-vaccination with IBD and 5 days post-vaccination with MD, then were monitored for 2 weeks, 4 days and 49 days, respectively, post-challenge to test the efficacy of the vaccination approach. Necropsy was performed for IBD- and MD-specific lesions. There were 138 birds used when testing efficacy against IBD and MD; for ND the number was 120.
In these tests, the researchers used both in ovo and subcutaneous routes of administration. Around 90% of US customers for the tested vaccines use in ovo, said Sing Rong, PhD, research director, Zoetis, while in Southeast Asia, the majority of producers use subcutaneous — though there is a current shift toward the use of in ovo machinery ongoing there too.
“There’s a market need to co-administer the vector vaccine with an immune-complex vaccine, as a single injection,” Rong said, explaining the rationale for the series of efficacy tests.
Strong protection
Protection against ND in the study testing the compatibility of Procerta HVT-ND and Bursaplex was 93% via the in ovo route and 95% via subcutaneous injection.[ii] Against IBD, both approaches resulted in 100% protection.[iii]
“Anything 90% or above is considered a full protection for ND and IBD. So, I think from this study, we conclude that if you co-administer the Procerta and the Bursaplex vaccines, you get the full protection for ND and IBD,” she said.
For MD, there was a difference in protection observed between application routes (97% for subcutaneous and 77% for in-ovo),[iv] but this could be attributed to the variability and complexity of the challenge model rather than vaccine performance, she explained. Anything 80% or above is considered full protection for MD.
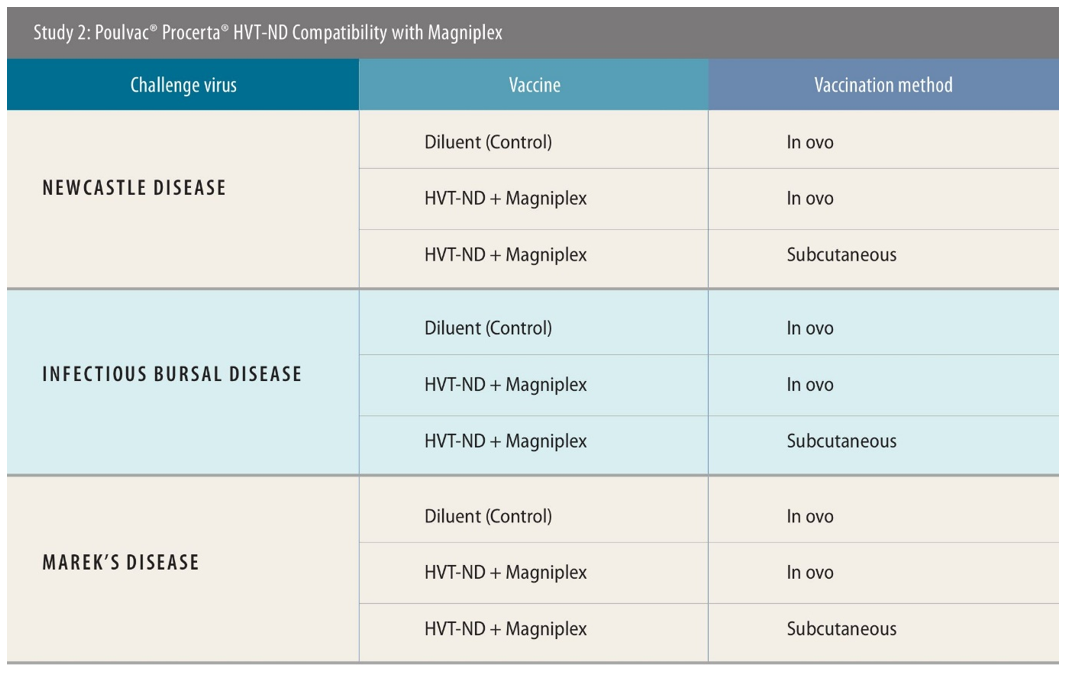
There were similar findings in the Magniplex studies.[v] ND efficacy was 97% and 100% for in ovo and subcutaneous administration, respectively,[vi] with 100% efficacy against IBD via both routes.[vii] For MD, the efficacy was 73% and 90% using in-ovo and subcutaneous application, respectively.[viii]
The Bursaplex and Magniplex vaccines use different IBD virus strains, and different global poultry markets tend to prefer one or the other.
Very virulent strains put combinations to test
Recombinant vector vaccines based on herpesvirus of turkey, such as Poulvac Procerta HVT-ND, have the added benefit of protecting against MD.[ix] However, producers can add MD CVI-988 strain vaccines when faced with severe MD challenges or when vaccinating long-lived birds such as layers and breeders.
In contrast to the first two studies, the third part was designed to incorporate the CVI strain vaccine into the vaccination strategy, alongside the vector vaccine alone, or also including Magniplex.
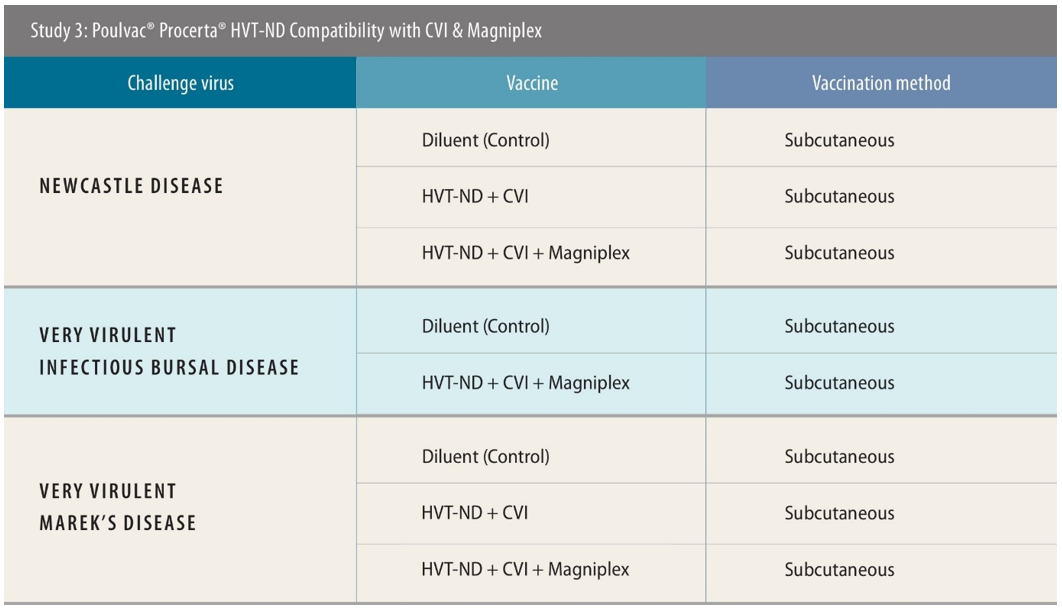
The challenge also differed, with birds challenged with very virulent IBDV and MDV (vvIBDV and vvMDV), as well as NDV. The scientists initiated the vvIBD challenge earlier for this part of the research — at 21 days of age — based on market demand for evidence of earlier protection.
In the vvIBDV and NDV aspects of the work, a total of 138 birds were used in each. Vaccines were administered subcutaneously, with the challenge virus introduced by eye drop in the case of vvIBDV and intramuscular injection for NDV. Researchers used 144 birds in the vvMDV testing, and the day-5 challenge was issued intra-abdominally.
The researchers observed 100% protection against ND using the two vaccines and 93% with the three.[x] Against vvMDV, the results were 89% with the two-vaccine approach and 96% with the three.[xi]
For vvIBDV protection, only the simultaneous use of Poulvac HVT-ND, Poulvac Ovoline CVI and Magniplex was tested. The researchers examined birds at both day 25 and 31, seeing 100% protection at both timepoints.[xii]
Validation matters for veterinarians
According to Rong, the results of these studies reassure poultry health practitioners that they can safely co-administer these vaccines while providing a high level of protection against three major poultry diseases.
“In all the studies, there was no safety issue. The hatch was very normal, and there were no clinical signs prior to challenge, showing a very good safety profile,” she said.
“There’s a wide need for simultaneous injection with these products to protect against IBD, Newcastle and Marek’s,” she added. “Having a solution, where you can have a single injection protecting birds from all three major diseases, that’s a big advantage for customers.”
| References | ||||
|---|---|---|---|---|
| [i] Data on file. Study Report No. B815R-US-18-A46, Zoetis LLC. | ||||
| [ii] Data on file. Study Report No. B81AR-US-19-A75, Zoetis LLC. | ||||
| [iii] Data on file. Study Report No. B81AR-US-19-A74, Zoetis LLC. | ||||
| [iv] Data on file. Study Report No. B81AR-US-19-B27, Zoetis LLC. | ||||
| [v] Data on file. Study Report No. B815W-US-21-E57, Zoetis LLC. | ||||
| [vi] Data on file. Study Report No. B81AR-US-19-B51, Zoetis LLC. | ||||
| [vii] Data on file. Study Report No. B81AR-US-19-B52, Zoetis LLC. | ||||
| [viii] Data on file. Study Report No. B81AR-US-19-B50, Zoetis LLC. | ||||
| [ix] Data on file. Study Report Nos. B911R-US-18-902, B911R-US-18-903, B911R-US-18-904, and B911R-US-18-906, Zoetis LLC. | ||||
| [x] Data on file. Study Report No. B815W-US-21-E57, Zoetis LLC. | ||||
| [xi] Data on file. Study Report No. B815W-US-21-E56, Zoetis LLC. | ||||
| [xii] Data on file. Study Report No. B815W-US-22-F65, Zoetis LLC. | ||||
| MM-27427 | ||||









