ProBe-Bac® PE: a powerful bacteriophage solution for safe and sustainable alternatives to AGP
New research on the latest bacteriophage solution from Pathway IntermediatesWith an eye toward the solution to problems on antibiotic resistance, many countries worldwide prohibited the massive use of antibiotic in treating diseases, or as growth promoters in livestock. The use of antibiotics was firstly reported in the 1940s, and considered an innovative discovery of the 20th
century. In spite of this, bacteria-causing diseases rapidly developed its antibiotic resistance after only a few years.
The World Health Organization (WHO) warned the public on the threat of using antibiotics in livestock feed. They stated that if antibiotics are incorporated in the animal feed, it could accumulate in their body which later adversely affects human immune system through the food chain. Meanwhile, the “Superbugs”, a multidrug-resistant bacteria, is threatening the world.
Banning the use of AGPs
In 2006, the European Union first banned the use of AGPs to reduce the use of antibiotics. Since the second half of 2011, South Korea also prohibited the use of all growth promoters. Meanwhile, the US Food and Drug Administration (FDA) proposed in 2017 the new guidelines in banning AGPs, allowing only those antibiotics prescribed by veterinarians for treating disease taking into consideration of animal health and welfare. Moreover, they warned that number of deaths attributed to Antimicrobial Resistance (AMR) will elevate up to 10 million by year 2050.
On the other hand, banning the use of AGPs in livestock feed could result to impaired growth rate, lower feed efficiency, and higher mortality. And in order to prevent this problems, an alternative to AGPs is necessary to improve animal growth performance, control pathogenic bacteria, and secure food safety.
Safe and sustainable alternatives to AGP
Antibiotic resistance has emerged as one of the major problems to principal public health. Actions in reducing and finally eliminating the AGPs use in livestock industry are mandatorily and voluntary expanding in order to diminish the risk of antibiotic resistance. However, these programs have provoked significant economic loss for producers and increased antibiotic used therapeutic treatments. Demands for AGP alternatives have become strong to resolve the global public health not only in combatting antibiotic resistant bacteria but to enhance the efficiency of therapeutic treatments. Thus, an alternative way to resolve this issue is very essential. Bacteriophage could be the answer to overcome this obstruction in the livestock industry. In fact, it is proven safe and sustainable alternatives to AGPs.
Bacteriophage: The next generation of alternatives to AGPs
Lytic bacteriophage is a microorganism that selectively kills bacteria in a species-specific manner, and to produce many of their own progeny, without causing any genetic mutation. The U.S. FDA has approved bacteriophage products as GRAS (Generally Recognized As Safe) substance.
ProBe-Bac is the latest bacteriophage solution
ProBe-Bac is the latest bacteriophage solution of Pathway Intermediates which was co-developed with Optipharm, an affiliate company of Pathway Intermediates and the leading company in the field of animal diagnosis and biomedical research. Pathway Intermediates aiming to provide a solution beyond controlling pathogens. It is a powerful combination of bacteriophages precisely selected against specific disease. This cocktail product allows to constraint several diseases that are rampant in poultry or swine industry. In comparison to existing bacteriophage products, the newly developed ProBe-Bac has improved stability and coverage which maximizes its efficiency in controlling bacterial pathogens. Bacteriophage colonizes the animal intestine and further controls the population balance of gut microflora. This could reduce the specific pathogenic microorganisms’ population resulting to improved feed efficiency and animal growth performance. Livestock animals under stress adversely affects the gut microflora of the digestive system which induces proliferation of bacterial pathogen, thus causing lower feed efficiency and diarrhea. Therefore, ProBe-Bac is developed to counteract these livestock industry obstacles.
ProBe-Bac PE is the product specifically for poultry which targets diseases such as typhoid, salmonellosis, and diarrhea. Upon ingestion, the bacteriophages from ProBe-Bac PE would settle in the animal intestine which inhibits growth of pathogenic microorganisms, improves the intestinal environment, and enhances feed efficiency.
Research on ProBe-Bac PE
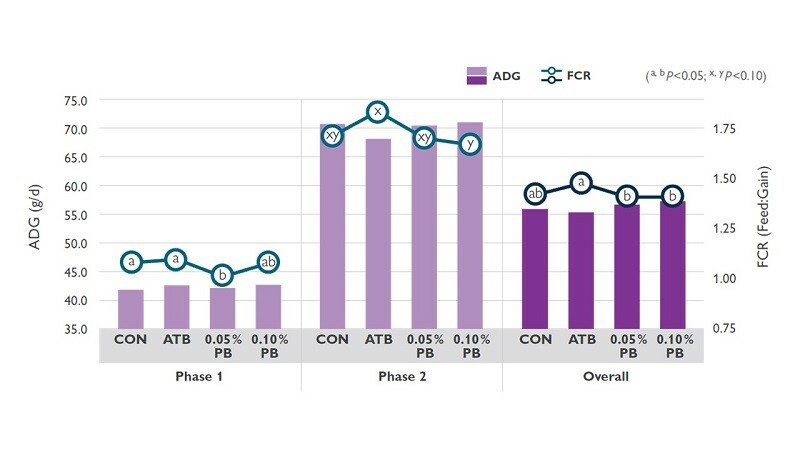
A recent study at Nong Lam University, Vietnam investigated the effect of ProBe-Bac PE supplementation on growth performance in broilers. The investigation used ProBe-Bac PE dietary levels of control (0%), 0.05%, or 0.10%. The evaluation was carried out with 480 day-old chicks (Ross 308; mixed sex). Treatments included CON (basal diet), ATB (basal diet + 0.01% Enramycin (EnradinF80)), 0.05% PB (CON + 0.05% ProBe-Bac PE), and 0.10% PB (CON + 0.10% ProBe-Bac PE). The experimental trial was conducted for 42 days (Phase 1 and Phase 2; 21 days/Phase). The data covered bird growth performance.
ProBe-Bac PE on growth performance of broilers
The feeding trial showed significantly improved FCR for the animal group that received different inclusion levels of ProBe-Bac PE (Figure 1). Specifically, during the Phase 1 of investigation, animal group with an inclusion of 0.05% PB to their diet had significantly lower FCR than that of CON and ATB. Assessing the result from the Phase 1 of feeding trial implies assurance of the beneficial effect of ProBe-Bac PE to broilers. Promisingly, Phase 2 and overall had a remarkable data showing a significantly lower FC for both inclusion levels (0.05 and 0.10%) of ProBe-Bac PE than the other treatments. Although, there were no significant differences among treatments on ADG, ProBe-Bac PE supplementation still had numerically higher daily body weight gain for the entire feeding trial, compared to other treatment groups. ProBe-Bac PE supplementation improved growth performance which was even better that antibiotic treatment. Overall, these results signify that ProBe-Bac PE could be suggested as an alternative to antibiotic contributing growth promotion in broilers.