



Role of Infectious Challenges in Nutritional Diseases
Infectious diseases result in important changes in nutrient absorption, metabolism and excretion, as well as exacerbating non-infectious diseases, according to Professor Kirk C. Klasing of the University of California, Davis.Speaking at the World Veterinary Poultry Association meeting in Cancun, Mexico in August, Professor Klasing said the net effect of a pathogen on the nutrient status of an animal is the sum of those direct effects resulting from local tissue damage and loss of tissue function plus the indirect effects that are secondary to the immune response.

The nutritional status of an animal is impacted by infectious diseases, stated Professor Klasing. Tissue specific pathology resulting from a pathogen can cause important functional changes that impact nutrient absorption, metabolism and excretion. For example, the negative effects of Eimeria acervulina on nutrient absorption can be severe. Even subclinical cases of coccidiosis or necrotic enteritis due to C. perfringens cause mild inflammatory damage to the intestinal mucosa leading to decreased digestion and absorption, reduced weight gain and poor feed conversion (Turk 1981; Elwinger, Schneitz et al. 1992; Waldenstedt, Elwinger et al. 2000; Tomar 2001; Annett, Viste et al. 2002; Van Immerseel, Rood et al. 2009).
In addition to the local pathological changes, the immune response to the pathogen orchestrates systemic changes of nutritionally important processes, continued Professor Klasing. These changes are somewhat 'generic' in that they are similar regardless of the causative pathogen and differ mostly in magnitude. The reason for this similar response is that most infectious challenges initially engage phagocytic cells and eventually shift to lymphocyte-mediated adaptive responses.
Phagocytes, including macrophages and neutrophils, initiate local and system inflammatory responses that are mediated by pro-inflammatory cytokines including interleukin 1, interleukin 6, gamma interferon, and interleukin 18. These cytokines can reach endocrine levels and reset the priority of virtually all physiological and developmental processes. These changes include the classical 'acute phase response' and include: decreased food intake; fever; hepatic production of acute phase proteins; impaired digestion and absorption of nutrients; changes in tissue priorities for uptake and use of nutrients; impairments in anabolic processes in skeletal muscle, bone, and many other tissues; diversion of nutrients to the tissues involved in immunity (Klasing 2005).
The net effect of a pathogen on the nutrient status of an animal is the sum of those direct effects resulting from pathology and loss of tissue function plus the indirect effects that are secondary to the immune response. Several important illustrations of this are provided below.
Inflammatory Responses in the Intestines Negatively Impact Intestinal Integrity and Function
Inflammatory responses, when sufficiently vigorous and prolonged, negatively impact intestinal integrity, stated Professor Klasing. Jeurissen et al. (Jeurissen, Lewis et al. 2002), define intestinal integrity as 'the cells and products constituting the barrier against leakage or translocation of feed components, microbial toxins, and microorganisms from the lumen to the body'. Integrity of the intestinal wall is primarily due to the continuous layer of epithelial cells that are tightly held together by adhesion molecules and by the layer of mucus on the apical surface of the epithelial cells. The amount of adhesion between epithelial cells and the amount of mucin is affected by the microbial milieu of the intestines and the diet (Smirnov, Sklan et al. 2004; Smirnov, Perez et al. 2005). Epithelial damage and inflammation diminish intestinal integrity, which allows the influx more microorganisms, which may amplify the inflammatory response.
Epithelial damage and inflammation decrease the digestive functions of the gut. Even mild infections of the intestinal epithelium result in marked impairment of nutrient digestion and absorption (Tomar 2001). This impairment is due to bolstered defensive processes, such as infiltration of lymphocytes into the epithelium, accelerated turn-over of epithelial cells, increased rate of peristalsis and profuse mucus secretion (Nusrat, Sitaraman et al. 2001; Tomar 2001; Schiffrin and Blum 2002).
In general, the absorption of micronutrients, especially fat-soluble vitamins and iron, are impacted to a greater degree than that of macronutrients such as carbohydrates, protein, or fat. Of the macronutrients, fat absorption is impacted most, according to Professor Klasing.
Three general mechanisms mediate the malabsorption. First, diminished absorption can be a direct result of pathological changes in the integrity and function of the intestinal epithelium. This is especially the case with infections that inflict pathology to the absorptive regions of the small intestines, e.g. Eimeria species. Second, malabsorption can be due to an increase in the rate of passage of digesta through the intestines because high rates of transient diminish the time available for digestion and absorption. Third, the reduction in absorption of some nutrients is orchestrated by the immune response to the pathogen. The changes in nutrient absorption that are mediated by the immune system may have protective value for the host by depriving microbes of nutrients that they need for proliferation (e.g. iron) or for defences against the immune system's effector mechanisms, e.g. antioxidants such as vitamin E and carotenoids.
Professor Klasing explained that the absorption of vitamin A and carotenoids are particularly decreased during enteric infections (Marusich, Ogrins et al. 1973; Allen 1992; West, Sijtsma et al. 1992). The magnitude of the decrease cannot be accounted for by a general decrease in lipid digestion and absorption. Diminished absorption of vitamin A and carotenoids is mediated by interleukin-1 and is a hallmark of the acute phase response to inflammation (Koutsos, Calvert et al. 2003). The absorption of iron is also markedly decreased during enteric infections. Some of this decrease is correlated with damage to iron-absorbing regions of the intestines (Turk 1981). However, pro-inflammatory cytokines mediate reduced iron absorption (Steele, Frazer et al. 2005) and are likely the primary mediator during many infections.
Decreased growth and egg production is a classic sign of most enteric challenges, including coccidiosis, necrotic enteritis, spirochaetosis, rotavirus infections campylobacteriosis, clostridial ulcerative enteritis and viral malabsorption syndrome (Saif 2008). Clearly, continued Professor Klasing, the reduced nutrient absorption caused by intestinal immune responses is responsible for much of the diminished performance. However, the acute phase response to intestinal inflammation also decreases performance especially because of its effect on appetite.
The impact of intestinal inflammation is shown in Figure 1, where specific pathogen-free broiler chicks were fed corn-based or rye-based diets, with or without dextran, which causes focal inflammation along the intestines (Gewirtz, Collier-Hyams et al. 2002). Rye is very high in non-starch polysaccharides, which cause apoptosis of epithelial cells leading to diminished intestinal integrity (Teirlynck, Bjerrum et al. 2009). The combination of rye and dextran leads to infiltration of lymphocytes between the columnar absorptive cells of the intestinal epithelium and cause decreased growth in chicks lacking known pathogens.
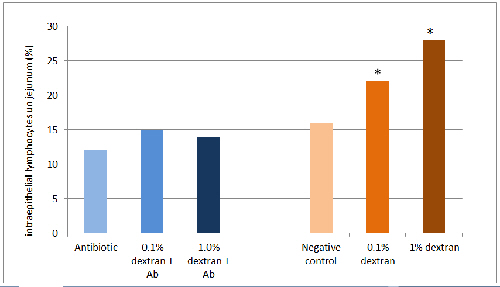
(Klasing and Peng, unpublished)
Teirlynck et al., (Teirlynck, Bjerrum et al. 2009) compared a maize-based diet to a wheat-rye diet for effects on broiler performance and intestinal morphology. Wheat-rye diet, presumably because of high non-start polysaccharides, caused villus fusion, a thinner tunica muscularis, T-lymphocyte infiltration into the epithelial layer, more and larger goblet cells, more apoptosis of epithelial cells in the mucosa and a shift in microbiota. Others have noted a thickening of the mucous layer and increased levels of inflammatory cytokines, such as interferon-γ. Non-starch polysaccharides induce proliferation of Clostridium perfringens (Annett, Viste et al. 2002), which induce epithelial cell damage and fusion of villi due to toxin production, and induce inflammation (Gholamiandehkordi, Timbermont et al. 2007). Furthermore, non-starch polysaccharides markedly increase the total microbial population (Choct, Hughes et al. 1996).
When chronic, impaired nutrient absorption increases the local inflammatory response, which further decreases nutrient absorption, said Professor Klasing. Although the intestines – by virtue of their first pass priority for nutrients – are not usually the primary site of pathology due to malnutrition, nutritional deficiencies impair the development of lymphocytes, especially T cells, which can result in diminished intestinal defences and increased incidence of pathogen-induced pathology and food induced intolerances. Furthermore, impaired T lymphocyte development shifts the burden of cell-mediated defence to phagocytes, which are more inflammatory. A vicious cycle often ensues with enteric infections causing malabsorption, which exacerbates nutritional deficiencies, which further compromises immunity. In field cases of stunting, runting, and unthrifty syndromes the causal trigger can be micronutrient deficiencies that cause immunodeficiencies (Klasing, unpublished field experiences).
Metabolic Diseases Exacerbated by Infectious Diseases
* "Birds that develop TD have better antibody responses to vaccination than those that do not." |
A variety of diseases of metabolic origin have emerged in the past few decades, stated Professor Klasing. Changes in genetics of poultry stock and their management are implicated in the increasing incidence of these problems. Intense selection of breeding populations for fast growth rates, high yield of edible products and efficient conversion of feed into body mass (especially skeletal muscle) has resulted in excellent productive characteristics of modern poultry. At the same time, physiological systems that support growth have not proportionally increased in size or capacity. In particular, the cardiovascular, pulmonary, hepatic, and skeletal systems are undersized and vulnerable to pathology in modern poultry. In broiler chickens, sudden death syndrome (flip-over) and pulmonary hypertension syndrome, which results in ascites, have emerged as economically important problems of the cardiovascular system.
Professor Klasing cited as examples ruptured aorta and cardiomyopathy, which cause sudden death and lead to high mortality in turkeys. Skeletal malformations, including tibial dyschondroplasia (TD), rickets and chondrodystrophy are also problems. The cellular and metabolic bases of many of these diseases have been described in detail but unfortunately, this understanding has not led to the eradication of metabolic diseases.
The systemic stress response associated with infection or trauma is emerging as an important factor that contributes to, and may sometimes be the primary cause, of the expression of several metabolic diseases, he said. For example, bacterial lipopolysaccharide triggers pulmonary hypertension, characterised by an increase in pulmonary arterial pressure similar to that seen in field cases of pulmonary hypertension syndrome (Wideman, Bowen et al. 2009). Additionally, the acute phase response causes startling large changes in bone metabolism and TD in broiler chicks. Within 48 hours after initiating an acute phase response by injection of lipopolysaccharide, bone mass decreases by 10 per cent and breaking strength by 20 per cent. Similarly, bone ash and bone calcium content are diminished. The loss in bone mass is much larger than can be accounted for by decreased growth rates caused by the acute phase response and represents mobilisation of existing bone. The growth plate is also markedly affected as indicated by a five-fold increase in the incidence of TD three days after initiating an acute phase response with lipopolysaccharide. The acute phase response also increases the severity of TD.
Interestingly, birds that develop TD have better antibody responses to vaccination than those that do not, continued Professor Klasing. They also develop a greater febrile response following a challenge with lipopolysaccharide. Yet, the mortality due to an intense acute phase response is lower in birds that develop TD. Apparently, the balance of the immune response between an adaptive antibody response and a systemic acute phase response differs between birds that are predisposed to TD and those that are not.
A chicken IL-1-like factor accelerates the rate of cartilage turnover, said Professor Klasing, citing his own research from 1994. This can be observed as an increase in proteoglycan release from cartilage taken from the growth plate of broiler chicks when incubated with chicken IL-1. IL-1 also activates osteoclasts and bone resorption. Presumably, the pro-inflammatory cytokines are responsible for the development of TD and loss of bone density and strength. The functional value of bone mobilisation and increased turnover of cartilage from the growth plate can only be speculated. Professor Klasing concluded that it may be that the remodelling of bone and the growth plate may be a necessary activity for immunesurveillance because these tissues are avascular and may be attractive areas for pathogens to reside and avoid immune defences.
References
- Allen, P.C. (1992). Effect of coccidiosis on the distribution of dietary lutein in the chick. Poult Sci 71(9): 1457-1463.
- Annett, C.B., J.R. Viste, M. Chirino-Trejo, H.L. Classen, D.M. Middleton and E. Simko (2002). Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol 31(6): 598-601.
- Choct, M., R.J. Hughes, J. Wang, M.R. Bedford, A.J. Morgan and G. Annison (1996). Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br Poult Sci 37(3): 609-621.
- Elwinger, K., C. Schneitz, E. Berndtson, O. Fossum, B. Teglof and B. Engstom (1992). Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Vet Scand 33(4): 369-378.
- Gewirtz, A.T., L.S. Collier-Hyams, A.N. Young, T. Kucharzik, W.J. Guilford, et al. (2002). Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol 168(10): 5260-5267.
- Gholamiandehkordi, A.R., L. Timbermont, A. Lanckriet, W. Van Den Broeck, K. Pedersen, et al. (2007). Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol 36(5): 375-382.
- Jeurissen, S.H., F. Lewis, J.D. van der Klis, Z. Mroz, J.M. Rebel and A.A. ter Huurne (2002). "Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr Issues Intest Microbiol 3(1): 1-14.
- Klasing, K.C. (1994). Avian leukocytic cytokines. Poult Sci 73(7): 1035-1043.
- Klasing, K.C. (2005). Interplay between diet, microbes, and immune defenses of the gastrointestinal tract. Consequences of Feeding in Vertebrates. J. M. Stark and T. Wang, Oxford University Press.
- Koutsos, E.A., C.C. Calvert and K.C. Klasing (2003). The effect of an acute phase response on tissue carotenoid levels of growing chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol 135(4): 635-646.
- Marusich, W.L., E.F. Ogrins, E. Schildknecht, P.R. Brown and M. Mitrovic (1973). The effect of subclinical infections with Eimeria praecox and Eimeria tenella on pigmentation and vitamin A absorption in broilers. Br Poult Sci 14(6): 541-546.
- Nusrat, A., S. V. Sitaraman and A. Neish (2001). Interaction of bacteria and bacterial toxins with intestinal epithelial cells. Curr. Gastroenterol Rep. 3(5): 392-398.
- Saif, Y.M., Ed. (2008). Diseases of Poultry, 12th Edition. Ames, Wiley Blackwall.
- Schiffrin, E.J. and S. Blum (2002). Interactions between the microbiota and the intestinal mucosa. Eur. J. Clin. Nutr. 56 (Supplement 3): S60-S64.
- Smirnov, A., R. Perez, E. Amit-Romach, D. Sklan and Z. Uni (2005). Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J Nutr 135(2): 187-192.
- Smirnov, A., D. Sklan and Z. Uni (2004). Mucin dynamics in the chick small intestine are altered by starvation. J Nutr 134(4): 736-742.
- Steele, T.M., D.M. Frazer and G.J. Anderson (2005). Systemic regulation of intestinal iron absorption. IUBMB Life 57(7):499-503.
- Teirlynck, E., L. Bjerrum, V. Eeckhaut, G. Huygebaert, F. Pasmans, et al. (2009). The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br J Nutr 102(10): 1453-1461.
- Tomar, B.S. (2001). Intestinal infections. Indian J Pediatr 68 Suppl 3: S8-18.
- Turk, D.E. (1981). Coccidial infections and iron absorption. Poult Sci 60(2): 323-326.
- Van Immerseel, F., J.I. Rood, R.J. Moore and R.W. Titball (2009). Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol 17(1): 32-36.
- Waldenstedt, L., K. Elwinger, A. Lunden, P. Thebo, M.R. Bedford and A. Uggla (2000). Intestinal digesta viscosity decreases during coccidial infection in broilers. Br Poult Sci 41(4): 459-464.
- West, C.E., S.R. Sijtsma, B. Kouwenhoven, J.H. Rombout and A.J. van der Zijpp (1992). Epithelia-damaging virus infections affect vitamin A status in chickens. J Nutr 122(2): 333-339.
- Wideman, R.F., O.T. Bowen and G.F. Erf (2009). Broiler pulmonary hypertensive responses during lipopolysaccharide-induced tolerance and cyclooxygenase inhibition. Poultry Science 88(1): 72-85.
Further Reading
| - | Find out more information on the diseases mentioned in this article by clicking here. |
November 2011








