Salmonellosis - clinical signs and prevention strategies in poultry
Description of clinical signs and prevention strategies for strains present in poultry worldwideIntroduction
Salmonellosis is a condition caused by infection with bacteria in the genus Salmonella (over 2,500 serotypes). There are two highly pathogenic serotypes of Salmonella which affect poultry and are common in some countries of South and Central America. However, since these serotypes are not prevalent in the US, we will focus on other strains of Salmonella which are present worldwide. These serotypes, although not highly pathogenic for poultry, have broad host specificity and thus have a tremendous relevance for public health. Therefore, this chapter will include advice for preventing the dissemination of poultry-derived Salmonella into the human population.
Etiology
Bacteria from the genus Salmonella are gram negative, non-spore-forming rods that can grow in a wide range of environments (variable oxygen concentration, high and low pH [4.0-9.0], variable temperature [from 36°F to 129°F], variable water activity [0.95-1.0]). Two strains, Salmonella Pullorum (pullorum disease) and Salmonella gallinarum (fowl typhoid), are non-motile and highly pathogenic for poultry, causing high mortality in both breeders and their progeny. In addition, these strains can affect many other species of birds and, rarely, some mammals. Fortunately, they are not present in Pennsylvania (PA). However, there are many other strains of Salmonella that are present in PA. Possibly the most relevant serotypes for public health are currently Salmonella enteritidis and Salmonella typhimurium. These motile forms of Salmonella, known as paratyphoid Salmonella, have very broad host specificity and can infect humans. In general, paratyphoid Salmonella are not highly pathogenic for most poultry. However, they survive and multiply well in poultry, which poses a great risk for human health. Unlike poultry, humans are susceptible to developing clinical disease in response to a Salmonella infection. Severe illness can occur in infants, elderly, and immunocompromised individuals.
Susceptibility of the agent
Several disinfectants can inactivate Salmonella when used as directed in their label. For example, phenolic and quaternary ammonium can inactivate Salmonella from various surfaces. Salmonella elimination can be more challenging when the bacteria are surrounded by organic matter. For example, Salmonella can be found in the fecal material of rodents, wild birds, and insects, where it has considerable protection from disinfectants. In this case, removal of organic material prior to disinfecting greatly increases the efficacy of the disinfection process. Salmonella can persist for a very long time in poultry litter (over one year). High environmental humidity increases the survivability of Salmonella. Composting the litter can eliminate all Salmonella from the litter; this requires temperatures of at least 55°C (131°F).
Salmonella is commonly found on raw feed materials (as well as complete poultry feed). In this case, aggressive thermal treatment (conditioning time) during pelleting normally destroys Salmonella. However, feed can (and does) get re-contaminated on its way from the feed mill to the farm. Salmonella-positive pellet coolers, conveyors, and feed trucks are common sources of feed re-contamination. In addition, when feed is left unprotected, wild birds, rodents, and insects can access the feed and introduce Salmonella. To protect the feed from re-contamination after thermal treatment, formaldehyde (1-2 kg/ton of feed), and to some extent organic acids (6-10 kg/ton of feed) have been used. In general, organic acids do not kill Salmonella in feed but can keep their numbers low. It is important to note that Salmonella can grow in a wide pH range (4.0 to 9.0).
Occurrence
Worldwide. Since Salmonella has broad host specificity, there are plenty of potential reservoirs which can be sources of infection. For example, humans, pets, rodents, wild birds, and insects (like flies and darkling beetles) can serve as vectors for introducing Salmonella into a flock. Breeders and hatcheries can be additional sources of contamination.
Pathogenesis
Most motile serotypes are not pathogenic for poultry under common circumstances. However, immunocompromised animals or very young animals may be susceptible to salmonellosis when challenged with very large doses of the bacteria. After a few days of life (perhaps due to the presence of competitive microflora in the intestine), the susceptibility of chickens to Salmonella infection is greatly reduced. Even very large doses of Salmonella (sufficient to kill 50% of day-old chicks) will not produce mortality in one-week-old birds.
Oral contamination may be the most common route for infection. However, it is also known that vertical transmission from mother to offspring occurs with some serotypes of Salmonella. The frequency of in-ovo contamination is fairly low; a more common infection route is the presence of Salmonella on the eggshell. Other routes of transmission, such as intracloacal and navel routes, are experimentally possible and may also be relevant in the field.
Once Salmonella reaches the intestine of young animals, it colonizes (with especial affinity for the epithelial cells in the ceca and ileocecal junction) and initiates persistent fecal shedding (for up to 6 months). Some serotypes (notably S. Enteritidis) can attach to and penetrate intestinal cells to gain access to the circulation, where they multiply. Bacteremia follows, and many soft and hard tissues can be infected by the bacteria (typically spleen, liver, and bones). Several strains of Salmonella are capable of multiplying in internal organs evading the immune system of the animals. After the initial bacteremia, it is possible to recover Salmonella from the birds for up to a year. The outcome of bacteremia depends largely on the initial dose and age at infection. If birds are infected with a moderate dose after one or two weeks of age, intestinal colonization and bacteremia can occur, but clinical signs are absent. Circulating antibodies are seen as early as 9 days post infection. It appears that humoral and cellular immune responses are needed to eliminate the bacteria from the host's tissues.
Clinical manifestations
For paratyphoid infections occurring after the first week of life, usually there are no clinical manifestations in poultry. However, if birds are infected during the first days of life with a very high dose of bacteria, clinical signs can be severe and are compatible with those of bacteremia: somnolence, ruffled feathers, anorexia, emaciation, dehydration, and diarrhea. The infection may result in stunting, blindness, lameness, and death. In addition to these clinical signs, S. arizonae can induce neurological signs in turkeys.
Necropsy findings
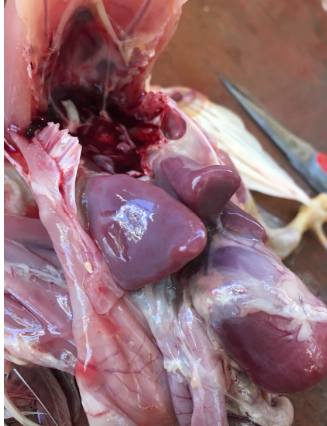
Lesions may appear in several organs due to bacteremia, which broadly distributes the organism in the tissues. Common findings are inflammation of the spleen (enlargement or splenomegaly) and liver (hepatomegaly, picture 1) along with peritonitis and/or pneumonia with airsacculitis (picture 2). In young birds, the yolk sac may remain unabsorbed and filled with caseous material. Necrotic lesions of the intestine are possible. Sometimes cecal cores (hard cores of fibrinous caseous material and cellular debris) are present. Arthritis and chondronecrosis are also found, but less frequently.
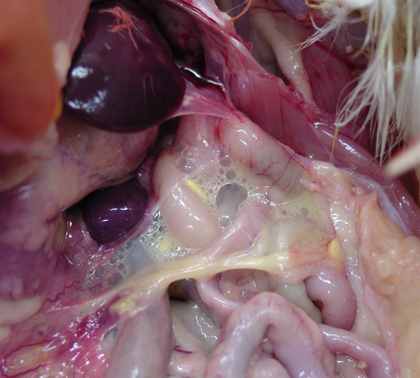
Diagnosis
The clinical signs and necropsy findings are not only produced by Salmonella. Many bacteria produce similar lesions. For this reason, direct culture of the organism from fecal material, intestinal tract, or internal organs is important. When the numbers of Salmonella are low, a pre-enrichment step is used to increase the number of viable cells before culturing them in differential media (such as tetrathionate broth).
Oftentimes, it must be known whether a particular flock is positive for Salmonella. For evaluating the flock as a whole, fecal material can be collected directly from the litter by dragging a moistened gauze across the floor. This gauze is later incubated in enrichment media, followed by culture in Salmonella-specific media. Dust samples are a good material for detecting the presence of Salmonella in the environment. Oftentimes, eggs are screened for the presence of Salmonella: the outside of the egg is washed, and the liquid derived from this process can be tested for the presence of Salmonella. The contents of eggs can also be tested for Salmonella. Even though Salmonella can be found inside eggs, the occurrence is very low, and sample size must be adjusted accordingly.
Relevant differential diagnosis
Bacteremia caused by other bacteria will show similar signs. Bacterial isolation is needed to confirm salmonellosis. Blindness can be caused by aspergillosis.
Prevention and treatment
Stringent biosecurity practices, covering all aspects of the operation, are needed to keep flocks Salmonella-negative. Salmonella cannot be eradicated from a flock with the use of antibiotics. Efforts must focus on preventing the entrance of Salmonella into a flock.
Effective prevention and treatment against Salmonella must start by obtaining eggs or birds from certified Salmonella-free flocks. If eggs will be incubated, the equipment and the eggs should be thoroughly disinfected. Before the arrival of new birds, the poultry house and equipment must be disinfected, and pests such as rodents and insects must be under control. Bait stations and traps must be operational for as long as poultry are present at the farm. Bait should be changed periodically as per the manufacturer recommendations.
Restrict the movement of equipment, personnel, and poultry between flocks. Water must be from a Salmonella-free source and treated (e.g., with chlorine). All feed should be given in pellet form and stored away from wild birds and rodents.
Water and feed acidifiers, probiotics, prebiotics, and yeast extract have been used as tools to decrease the ability of Salmonella to colonize the intestinal tract of poultry, with limited success. In general, these strategies have shown some efficacy reducing the intestinal colonization and organ invasion after a challenge. However, the contamination is never eliminated.
Vaccines against S. enteritidis has been used in layers to reduce the susceptibility of the animals to a Salmonella challenge. This approach, coupled with stringent biosecurity standards, has worked in European markets to reduce the incidence of S. enteritidis.