



Study of inactivated S. Enteritidis vaccine against S. Gallinarum
Efficacy study of an inactivated Salmonella Enteritidis vaccine against Salmonella Gallinarum challenge in commercial layersAs long as the world population grows, so does the food supply industry. During the last 20 years, the poultry population has grown by over 35%. Furthermore, year by year, layer genetics companies are improving performance in the later stages of life, producing a high profitability bird kept on the farm for over 110 weeks. However, this fact increases the risk of them being challenged by different infectious agents, including viruses, bacteria and parasites, as the pen is not cleaned for longer periods of time. Amongst all the bacterial diseases that threaten poultry livestock, there is one that focuses special attention on prevention in both poultry health and human health: Salmonellosis.
It is well known that there are a large number of serotypes that belong to this family of enterobacteriaceae, which has over 2,500 different serotypes. Two groups can be distinguished, a group that affects both birds and mammals, the broad-host species, including Salmonella Enteritidis and Salmonella Typhimurium, and a second group of a few serotypes that only infect birds. These are the host-specific ones, such as Salmonella Gallinarum and Salmonella Pullorum. Focusing on the second group, one of the main differences compared to broad-host serotypes, is their great capacity to produce severe lesions, including liver, spleen and ovarian dysfunction, which is the reason why Salmonella Gallinarum and Salmonella Pullorum are two of the 10 most harmful poultry diseases.
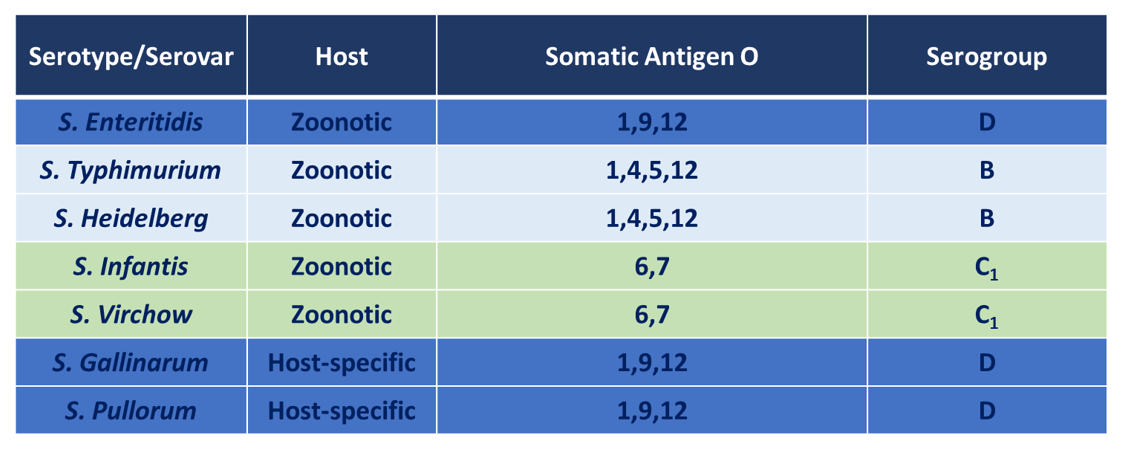
From an immunological point of view, as previously described, the natural behaviour of the Salmonella genus is facultatively anaerobic, meaning that both live and inactivated vaccines play a key role in reducing intracellular and extracellular Salmonella. Currently, inactivated Salmonella Enteritidis vaccines are used for a Food Safety purpose, but also to boost the protection offered by live SG9R vaccines, extending the duration and potency of live SG9R vaccines. Many authors in the past (Paiva, JB., Penha Filho. R., Arguello,Y.,) have described the serological relationship between SE and SG, as both serotypes share same somatic antigenic formula (O: 1,9,12), so that the antibodies produced after delivering an inactivated Salmonella Enteritidis vaccine would cross-react against different serotypes belonging to the same serogroup D, giving cross-protection to other serotypes such as Salmonella Gallinarum and S. Pullorum. This a widespread practice used in South America, Asia and Eastern European countries with practical benefits, but has not yet been sufficiently scientifically studied.
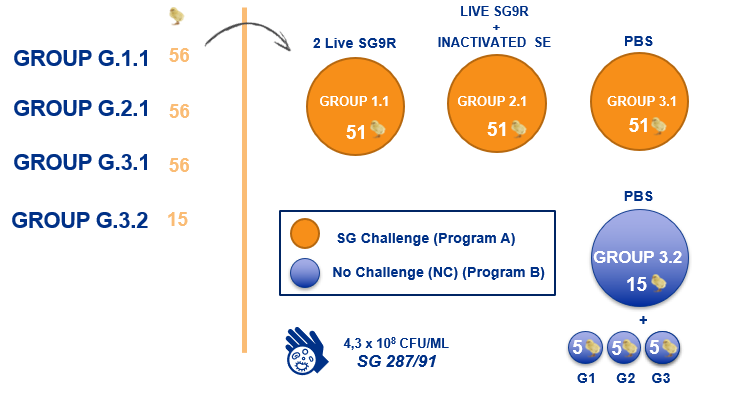
Due to all the above, a study was conducted to investigate the efficacy of different vaccination programmes, including SE-based vaccines, in controlling the systemic infection caused by an experimental challenge with Salmonella Gallinarum in layer hens. One-day-old brown layer hens (Hy-line) were used in the study and randomly distributed into 3 groups (G1-G3) with 56 animals in each. The groups were initially placed in separate rooms and reared in cages containing 5 birds each. G1 received 2 doses of a commercial live SG9R vaccine at 6 and 14 weeks, G2 received a commercial live SG9R vaccine at 6 weeks and an inactivated SE vaccine at 14 weeks, G3 did not receive any vaccine (control group). Before the challenge, the birds in each group were separated into 2 subgroups of 5 (G3.2) and 51 (G1.1-G3.1) animals each. At 20 weeks of age, group G3.2 was given PBS by oral gavage and groups G1.1-G3.1 were infected with Salmonella Gallinarum (4.3 108 CFU/bird). Mortality, clinical signs, humoral response and egg laying rate were evaluated daily up to 5 weeks whilst lesions and bacterial load in the liver, spleen and ovary were monitored weekly up to 3 weeks post challenge.
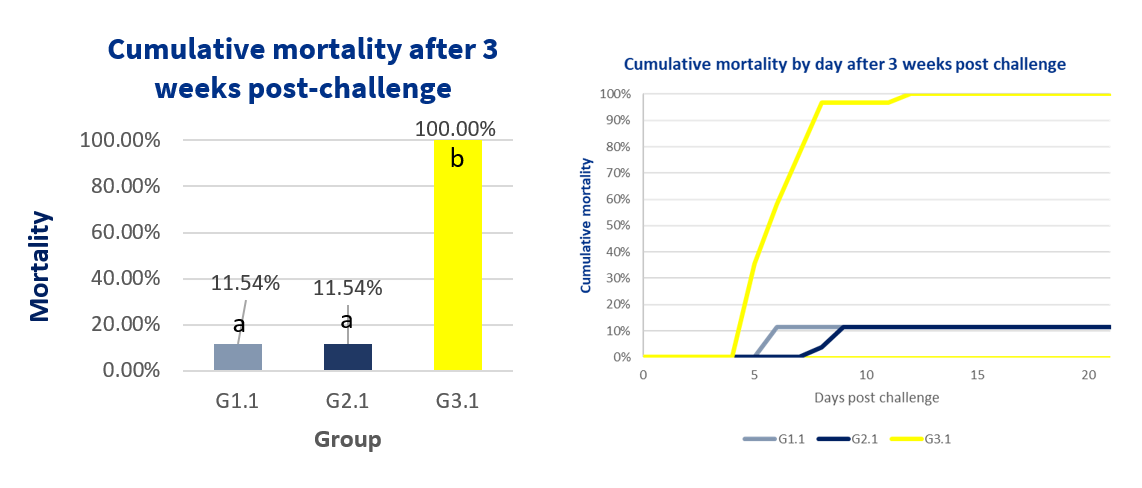
The challenge produced mortality, clinical signs, and organ lesions in all the non-vaccinated birds (G3.1); in contrast, none of the birds in the non-challenged group (G3.2) developed any of these. Groups G1.1 and G2.1 showed a statistically significant lower mortality rate (11.5%) compared to G3.1 (100%) (Fisher exact test; p<0.05).
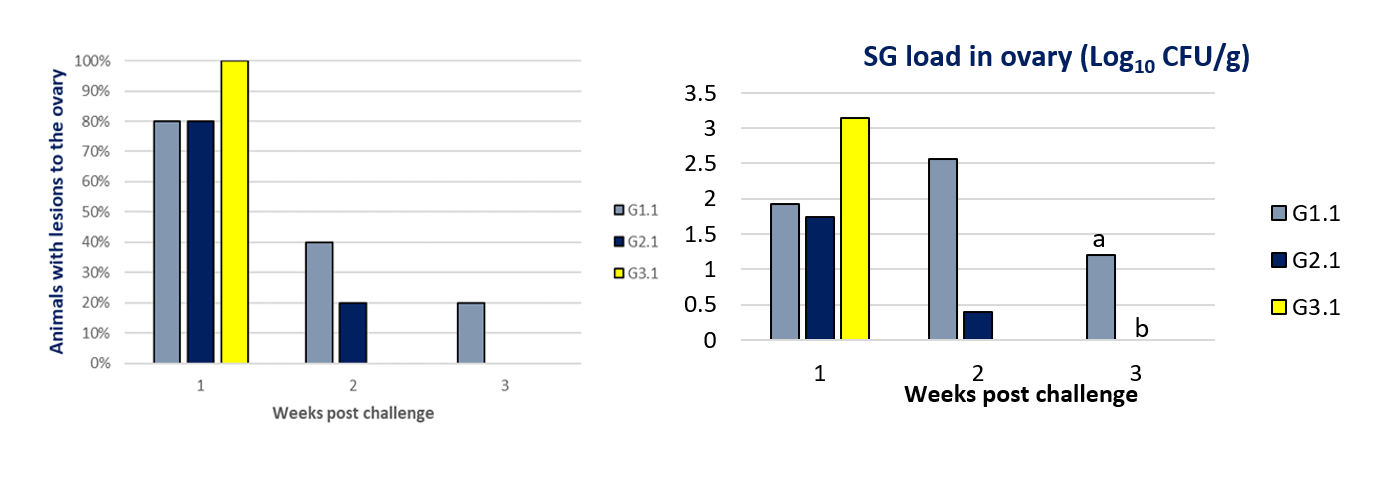
Moreover, in the case of the post-mortem lesions in the target organs after the challenge, a significantly faster reduction in the lesions was observed in ovarian samples in group G2.1 (0% of birds with post-mortem lesions at 21 days post challenge) compared to group G1.1 (20% of birds with post-mortem lesions at 21 days post challenge). Furthermore, in the ovary, a significant difference (p value < 0.05) was also observed between group G1.1 and group G2.1, showing a faster clearance of bacterial count in the birds vaccinated with the combination of live SG9R with the inactivated SE vaccine (G2.2), in which no bacteria were found at 21 days post challenge.
From the serology samples, after the challenge, there was an increase in serogroup D antibodies, reaching 10,000 units in all groups and showing no statistical difference between the challenged groups at 3 weeks after the challenge. Nevertheless, a significantly faster antibody peak (p value < 0.05) was reached by group G2.1, one week after challenge, whilst group G1.1 took 3 weeks to produce the peak of antibodies.
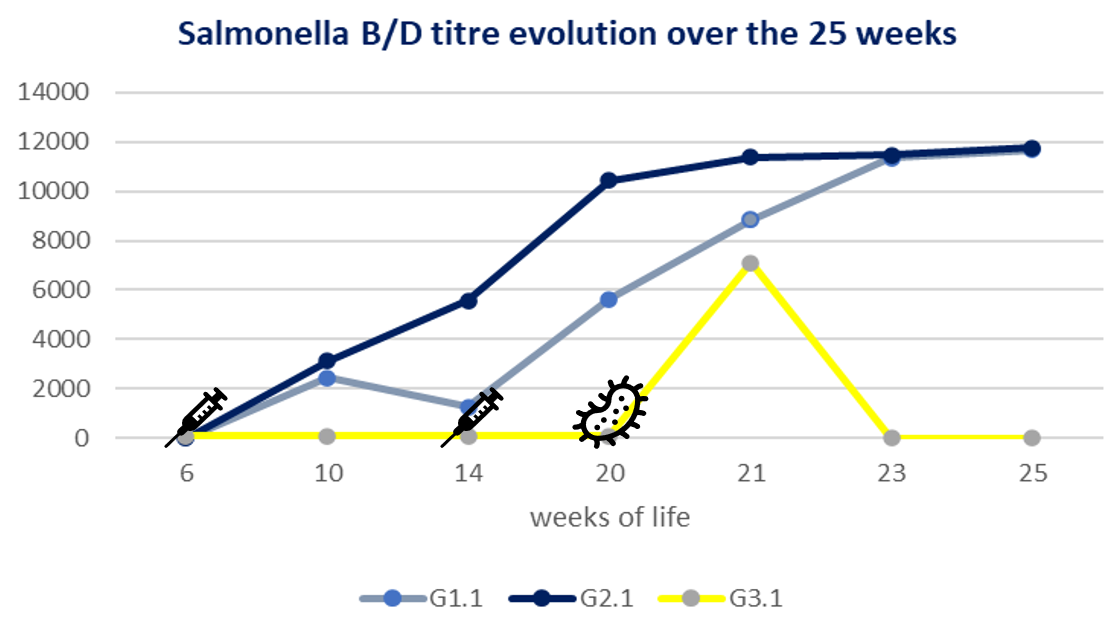
Finally, the egg laying rate was studied with the aim of assessing the impact of a Salmonellla Gallinarum challenge during the start of lay. Unchallenged group G3.2 represented healthy birds with a standard laying profile. Birds in group G2.1 had a higher peak of production after recovering from the challenge than birds in group G1.1. These findings suggest a faster ovarian recovery as a result of protection of this organ with a high level of circulating antibodies.
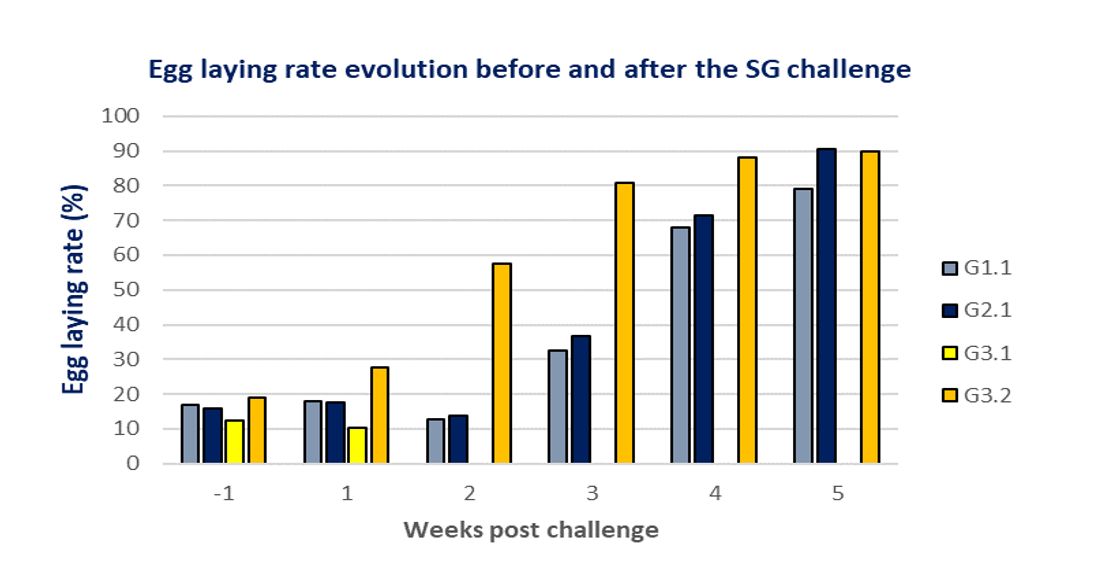
Based on these results, it can be concluded that over a short-term period, the combination of a primary live SG9R vaccine followed by a booster dose of inactivated SE vaccine can improve the protection against a Salmonella Gallinarum challenge by reducing mortality in challenged commercial layer hens and shorten the bacterial clearance period in internal organs, such as the ovary, thereby reducing the chances of vertical transmission and enhancing protection of the offspring. Further studies must be carried out on a long-term basis to increase our knowledge of Salmonella Gallinarum behaviour and immunology.









