Technical update: Infectious Laryngotracheitis (ILT)
Infectious laryngotracheitis (ILT) is a viral respiratory disease caused by an herpesvirus of chickens and some other gallinaceous birds (e.g. pheasants, peafowl). It is an economically significant disease in commercial egg producing flocks, with an essentially global distribution. In regions where ILT is endemic, the commercial poultry industry is faced with multimillion-dollar losses as a result of mortality, egg production losses, and decreased bird growth [1].Clinical signs of ILT
Disease caused by ILT manifests primarily as injury to the upper respiratory tract. Affected flocks exhibit depressed feed consumption followed by egg production loss and increased mortality. In a severe, acute ILT challenge, almost all birds (90– 100%) in a flock may show some sign of disease.
Clinical signs are associated with the bird’s attempt to clear obstruction of the trachea or larynx with plugs of mucus and/or blood. These signs include bloody nasal discharge, head shaking, and soiling of back and wing feathers with mucus and blood. Birds with respiratory distress will gape, gasp, and cough with moist rales and extend the neck to breathe. In more severely affected birds, the comb and wattles appear dark due to hypoxia (low blood oxygen). Conjunctivitis is commonly observed. Flock mortalities will typically range between 10– 20%, although mortality as high as 70% occurs in severe outbreaks. Sudden death from asphyxiation with no clinical signs occurs as a result of complete tracheal and laryngeal obstruction [1].
Clinical presentation of ILT within a flock will vary depending on the virulence of the challenging viral strain, and location of initial viral contact [2]. The course of disease also varies by pathogenicity of the viral strain. Flocks infected with milder virus strains may recover within as little as 10 days, while recovery from more pathogenic strains may take as long as 4 weeks.
Figure 1. Chicken with neck extended, demonstrating respiratory distress. Photo: Dr. Robert Porter, Jr., University of Minnesota.
Signs of disease in subacute outbreaks may resemble those of an acute event, but with a slower progression of disease, and mortality on the lower end of the spectrum (10–30%). Post-mortem findings are generally less marked in these instances. In very mild outbreaks of ILT, clinical signs are generalised and may include tearing, conjunctivitis, infraorbital swelling, nasal discharge, depressed egg production, decreased feed intake, and weight loss [1].
Incubation period
Typically, clinical signs of ILT appear 6–12 days after infection. The virus is usually shed in respiratory secretions for at least 6–8 days following initial infection. Shedding may continue at a reduced level for as long as 10 days. The virus then may move to nerve ganglia to become a latent (silent) infection, where the virus can remain for months in the bird [1].
Viral Latency
Like all herpesviruses, the ILT virus can reside for long periods of time in the host’s nerve tissue following initial inoculation. While in this state of latent infection, birds will not exhibit signs of disease, nor will they shed virus. Stress events such as transfer of birds or onset of lay can trigger reactivation of the virus and cause disease and viral shedding. Flocks infected with ILT are assumed life-long carriers of the virus and a source of future infections for other flocks [3].
Transmission
Birds are infected with ILT by exposure to respiratory exudates of infected birds. The virus gains entrance into the bird’s body through the upper respiratory tract and eyes. ILT virus has been shown to infect birds via inhalation into the trachea, or via contact with the mucosal tissues of the eyes or nasal cavity. The virus may also be passed from the oral cavity into either the nasal cavity or trachea [2].
The virus may also be introduced onto a poultry facility with contaminated clothing, footwear, vehicles, beak-trimming machines, vaccination tools, or other equipment contaminated with soil, manure, and bird tissues. People performing beak trimming, vaccination, or moving birds are a particular risk to cross-contaminate ILT into clean houses. Testing of dust from exhaust fans has detected infectious virus as far as 500 m from a contaminated barn [4].
Once a facility is infected, transmission occurs primarily on a bird-to-bird basis. Acutely infected and diseased birds shed virus and pass disease more readily than clinically recovered carrier birds. Transmission may occur at the same rate within cage or cage-free housing types. In cage housing, a clear pattern of disease progression through the flock may be observable, radiating from the probable initial infection. Multi-age laying farms provide an environment with high potential to spread the virus from older infected flocks to younger susceptible flocks [3].
Susceptibility to disinfection
The ILT virus is an enveloped virus, making it vulnerable to many common commercial chemical disinfectants, including those with a lye, cresol, hydrogen peroxide, halogen-detergent, or iodophor base. The virus may also be rapidly inactivated (48 hours or less) in the environment under direct exposure to sunlight and/or high temperatures (38°C/100.4°F). In dark, damp, cool conditions, the virus may persist in organic material for as long as 100 days [1].
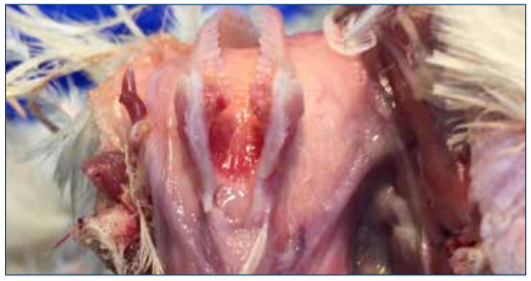
Figure 2. Inflammation of the larynx and trachea.
Post-mortem lesions
As with clinical signs, post-mortem lesions of laryngotracheitis may vary with severity of disease, and the location of initial introduction of the virus into the host’s body.
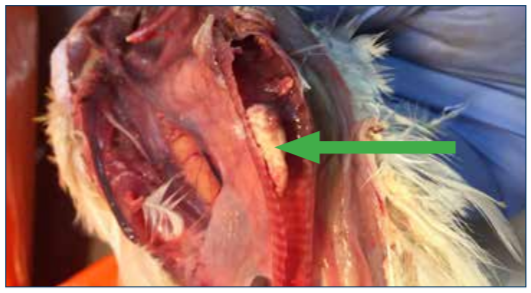
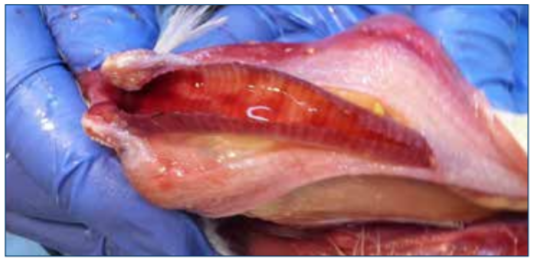
The hallmark lesion in severe cases of ILT is a hemorrhagic inflammation of the trachea and the presence of bloody mucoid casts that extend the length of the trachea. Inflammation of the respiratory and conjunctival mucosal surfaces may be observed as well. The disease progression required for formation of tracheal casts is long enough that some birds may die acutely, especially early in an outbreak, with no gross pathologic signs. In mild cases of ILT, or early in an outbreak, pathologic findings may be limited to swelling of the conjunctiva and infraorbital sinuses [1].
Diagnosis
Clinical signs and necropsy lesions can be suggestive of ILT but cannot be differentiated from other diseases with similar presentation. The main differential diagnoses are Newcastle disease, infectious bronchitis, avian influenza, and wet pox. In particular, the clinical and post-mortem signs of ILT and wet pox may appear identical.
Figure 3. Fibrinohemorrhagic tracheitis characteristic of ILT. This plug may occlude the trachea and cause death from asphyxiation.
Figure 4. Hemmorhage within the trachea is a common sign of infection.
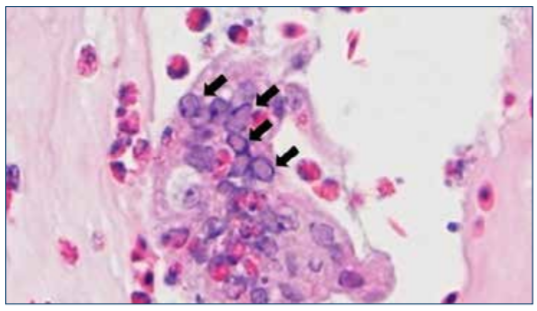
Definitive diagnosis of ILT is made by the microscopic examination of tissues of the eye lid (conjunctiva) and trachea from affected birds. A positive identification will demonstrate intranuclear inclusion bodies characteristic of ILT. Intranuclear inclusion bodies appear starting at about 3 days post-infection and are only present for up to 5 days. Due to the course of disease, these cells necrose and shed. As a result, early observation and astute collection of birds for sampling is important for diagnosis. Live chickens selected for sampling should be euthanized with carbon dioxide or other poultry-appropriate combinations of gas, rather than by cervical dislocation, to avoid damage to the trachea. Birds can be submitted live or freshly dead to the lab, or tracheal tissue can be collected in the field and submitted in formalin. If tracheal tissue is submitted, it should be cut in whole sections 2–3 cm (1 in) long where the lumen of the trachea is undisturbed for the most accurate histological observation.
Laryngotracheitis virus can also be detected from live bird samples in exudate from the conjunctiva or respiratory tract, in cell culture, or via serology. Virus isolation and PCR via tracheal swab, and ELISA via serum are highly sensitive for the detection of the virus or infection. Serological testing by IFA or ELISA can provide rapid results, with ELISA being the less subjective of the two tests [1].
Figure 5. Microscopic view of affected tracheal mucosa. Large multinucleated synctial cell showing many intranuclear inclusion bodies (arrows). Photo: Dr. Yuko Sato, Iowa State University.
Intervention strategies
Vaccines
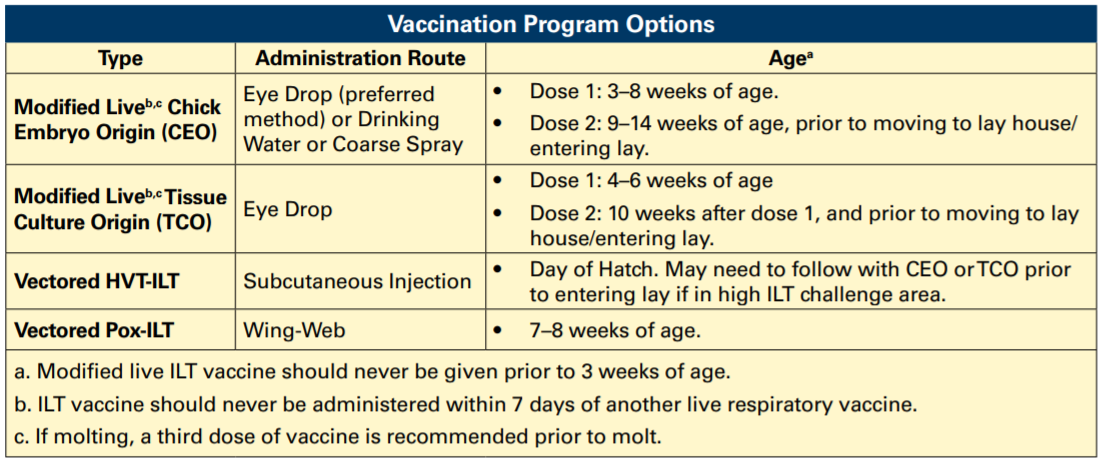
Vaccination for ILT cannot prevent infection; however, in areas where the disease is endemic, vaccines can protect against the clinical effects of the disease, including production traits. Presently available vaccines include:
- CEO (Chicken Embryo Origin): Modified-live virus vaccine delivered via water lines, spray, or eye drop.
- TCO (Tissue Culture Origin): modified-live vaccine delivered by eye drop.
- vPox-ILT and vHVT-ILT: ILT gene fragments are vectored into either pox virus or HVT virus and applied by wing web or injection.
Revaccination in response to an outbreak
Vaccinating flocks during an ILT outbreak can be effective in reducing the spread of the disease within a flock. Revaccination works because the spread of the disease throughout the house can be slow. There is not enough time to handle all of the birds for an eye drop vaccination, so spray or water application is the best method. Spray vaccination can be accomplished very quickly, but there is a greater risk of rolling vaccine reactions in the vaccinated house and in adjacent houses. Water vaccination requires two consecutive doses (one-hour doses back to back). This method has been observed to be more effective and less reactive than spraying.
ILT outbreaks of vaccine origin
Historically prevalent modified-live CEO vaccines for ILT, administered through water lines or by spray, are attractive. They provide a much more expedient and less labour intensive alternative to individual vaccination via eye drop (CEO or TCO modified-lived ILT vaccines), or direct injection (vectored vaccines). However, mass vaccination via water or spray carries a well-documented risk of unwanted spread of the vaccine virus and potential for disease. Poor water or spray vaccination technique which has left many birds unvaccinated can allow bird-to-bird passage of the vaccine strain. This may cause the vaccine virus to become more pathogenic to susceptable birds. The spread of the vaccine virus also occurs when not all farms in the area are using ILT vaccination. The result is spread of the ILT virus and increased virulence. Many ILT outbreaks are due to "vaccinal laryngotracheitis." In some regions, this risk has led to legal restrictions on the use of live ILT vaccines [3].
Management
Stringent biosecurity is effective as a preventive measure against ILT. Sanitation of people, equipment, and vehicles should be practiced to minimise the risk of carrying infected material into contact with the flock. Controlled movement of personnel and chickens is as important as ever in stemming the spread of disease. Deliveries of feed, birds for placement, and other essentials should be routed to avoid passing other commercial facilities with a history of ILT, those using ILT vaccine, and sites containing backyard flocks. Mixing of birds of different ages should be avoided whenever possible to minimise the risk of disease passing from infected or vaccinated birds to naïve birds [1].
Disease control and eradication programs for ILT have been attempted in multiple regions. Success has depended heavily on cooperation among commercial poultry raisers to rapidly identify cases, coordinate movement, utilise vaccination strategically, negotiate indemnity with local authorities, and outreach to local backyard and hobby flocks that may serve as a reservoir for reinfection.
Thorough cleaning and disinfection of facilities following depopulation, and extended downtime before repopulation, have also shown merit as a means of eliminating circulation of LTv both on individual sites, and as part of regional control efforts [5].
Treatment
No effective treatment for laryngotracheitis exists at this time.
References
1. Guy, James S. and Trevor J. Bagust. Chapter 4: Laryngotracheitis. Diseases of Poultry. 13th edition. Ames: Wiley-Blackwell, 2013. Print.
2. Beltrán, Gabriela et al. The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine. Avian Pathology. 2017. Online Edition.
3. Dufour-Zavala, Louise. Epizootiology of Infectious Laryngotracheitis and Presentation of an Industry Control Program. Avian Diseases. 2008; 52:1-7.
4. Volkova, Victoriya et al. Factors Associated with Introduction of Infectious Laryngotracheitis Virus on Broiler Farms During a Localized Outbreak. Avian Diseases. 2012; 56: 521-528.
5. Chin, R.P. et al. Intervention Strategies for Laryngotracheitis: Impact of Extended Downtime and Enhanced Biosecurity Auditing. Avian Diseases. 2009; 53:574-577.