



Vectormune ND protects against Indian genotype XIII of Newcastle disease virus
Vector rHVT-F vaccine protects chickens and reduces shedding against an Indian genotype XIII Newcastle disease virusRanikhet (Newcastle) Disease is a highly contagious viral infection that affects a very broad range of birds, especially chickens and other poultry species. It is caused by Newcastle Disease virus (NDV), which was recently renamed as Avian orthoavulavirus serotype 1 of the Orthoavulavirus genus, Avulavirinae subfamily and Paramyxoviridae family. It can cause different clinical signs and lesions depending on the virulence of the virus and the susceptibility of the host. Additionally, Newcastle Disease due to velogenic NDV strains is a notifiable disease, and it is one of the main barriers for international trade of poultry and poultry products.
The early techniques to classify the NDV were mainly based on its biological properties, such as pathogenicity, plaque formation, thermostability, analyses of structural polypeptides, and hemagglutination inhibition patterns using monoclonal antibodies (Ballági-Pordani et al., 1996; Russel and Alexander, 1983). With the increasing use of molecular techniques in viral research, the knowledge of the NDV genetic characteristics improved significantly and several molecular classification systems have been successively proposed. They targeted the surface F (fusion) protein gene sequence.
In 1996, Ballági-Pordani and co-workers suggested the classification of NDV isolates into six distinct genotypes (I to VI) based on restriction fragment length polymorphism (RFLP) analyses. This system was validated and further improved by phylogenetic analysis of partial F gene sequence data, and additional NDV genotypes have since been identified (Herczeg et al., 1999; Lomniczi et al., 1998). Diel et al. (2012) proposed a unified and objective system based on the phylogenetic topology, evolutionary distances, branch support and epidemiological independence. It became widely used and resulted in the identification of new genotypes. However, the uncoordinated naming, insufficient application of the criteria, incorrect assignments, using limited/partial datasets called for another revision and a consortium of 29 World Organization of Animal Health reference laboratories for ND was established in 2014 to revise NDV classification system. Dimitrov et al. (2019) published the results of such effort with an updated classification system that has a total of at least 20 distinct genotypes (I to XXI), including the three new class II genotypes.
The genotype XIII and its subdivisions have been detected in India, Bangladesh, Pakistan, Iran, but also in more remote places like South Africa, Zambia, Tanzania, and others. These viruses can cause high mortality with massive haemorrhages, congestion and necrosis in different visceral organs, and lymphoid depletion (Khorajiya et al., 2015; Nooruzzaman et al., 2021). In India, the genotype XIII has apparently become the most prevalent strain causing enormous economic problems to local producers. The mortality rate caused by NDV genotype XIII, with or without co-infections with other respiratory pathogens, in layers, colour broilers & backyard chickens, can reach up to 40 – 50% (Khorajiya et al., 2015; Gowthaman et al., 2019).
With such massive impact on the Indian poultry sector, researchers, opinion leaders and producers have been looking for ways to protect flocks against the negative consequences of this infection that certainly involve adhering to strict biosecurity protocols and revising vaccination programs. This short article summarizes the first ever challenge study using an Indian genotype XIII NDV strain in chickens that were immunized with a vector rHVT-F vaccine.
The vector rHVT-F vaccine
The vector vaccine of the rHVT-F type, commercially known as Vectormune ND, uses the herpes virus of turkeys (HVT) as the backbone and in which genome the Fusion (F) gene of a genotype I, D-26 strain of NDV has been inserted. Its design is unique in terms of insertion site, promoter, and origin of F gene. The HVT strain used to carry and express the F gene has been known for decades as a very safe and stable virus, used worldwide to vaccinate chickens against Marek’s disease. The strain (FC-126) and passage level selected for the construction of Vectormune ND ensure an active replication in chickens and a strong expression of the F gene which are key points when it comes to early onset of immunity and high protection rate.
The F protein is the main virulence factor of NDV; indeed, it is present on the surface of NDV, allowing it to attach to and penetrate the target cells. It is at the same time a key immunogen. In this way, if immunity is built up against F protein, then NDV can no longer infect cells and create damage.
The protection induced by Vectormune ND against different genotypes has previously been assessed by challenge studies conducted in commercial broilers, layers, turkeys, and SPF chickens using genotypes II, III, IV and sub-genotypes V.2, VII.1.1, VII.2, VIII and XII.1 (according to the latest classification as proposed by Dimitrov and co-workers). In all cases, clinical protection reached up to 90 – 100% with a significant shedding reduction (Paniago et al., 2016). This preliminary data provided strong evidence of the flexibility of this vaccine construct to cope with a wide range of NDV strains, belonging to a wide array of genotypes. The consistency of clinical protection and of significant reduction of virus shedding are critical in the long-term prevention of this deadly disease. Because of the detection and wide circulation of genotype XIII in India and in several other countries, it became relevant to test the efficacy of Vectormune ND against it, since it has never been done before.
Materials and methods
The study was conducted at the Indian Institute of Technology (IIT), Guwahati, Assam, in collaboration with the Center for Medical Biotechnology of the Maharshi Dayanand University (MDU), Rohtak, Haryana, India. Briefly, one-day old commercial broilers, Cobb 430 breed, with moderate level of maternally derived antibodies against NDV of 7.5 log2 (HI titer) or 11,179 ELISA units (BioChek ELISA kit), were allocated in two groups. Twenty-two (22) birds were vaccinated at hatch with the rHVT-F vaccine (0.2 ml, by subcutaneous route), whereas 10 birds were kept as unvaccinated controls. No other ND vaccines were used.
At 4 weeks of age, all birds were challenged using an Indian genotype XIII NDV strain, NDV/Chicken/Pandu/2015 (GenBank accession number KY774445), at a dose of 102 CLD50 /bird (0.1ml), by oculo-nasal route and they were daily monitored for 12 days after the challenge. Oropharyngeal swabs were taken at 2- and 4-days post-challenge (dpch) and the shedding was assessed by hemagglutination activity (HA) in SPF embryonated eggs.
Results
The vaccine induced 95% of protection against mortality and morbidity while all birds in the control group succumbed with the characteristic signs and lesions of NDV infection within 6 to 9 days (Figure 1).
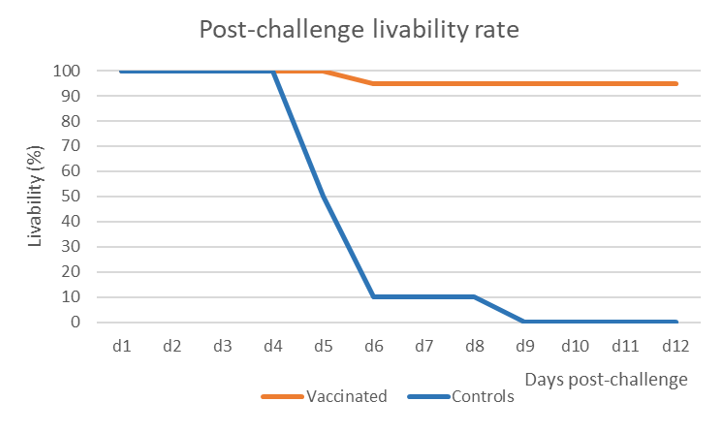
Oropharyngeal swabs were taken at 2- and 4-days post-challenge (dpch) and the shedding was assessed by hemagglutination activity (HA) in SPF embryonated eggs. This technique does not only detect the virus, but it also assesses if the detected virus shed by the birds is still alive, contrary to the PCR which only detects nucleic acid. Serial dilutions of the inoculum in eggs enable quantifying the HA activity and the results are expressed in Figure 2.
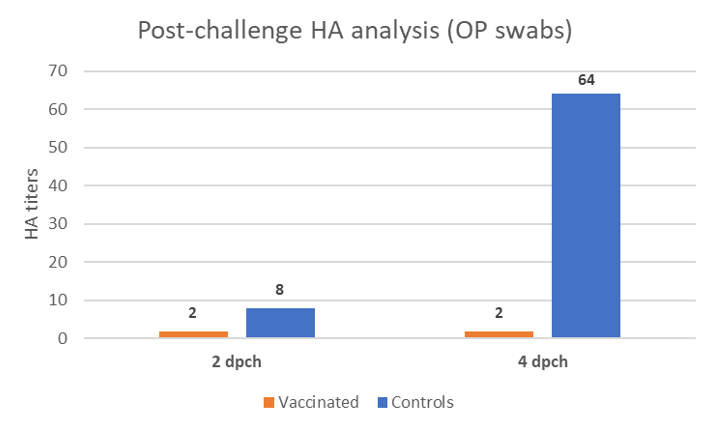
At 2 dpch, birds vaccinated with the rHVT-F vaccine already showed a slight reduction in the re-excretion of the challenge virus by the oropharyngeal route. At 4 dpch, chickens from the control group shed considerably higher amount of virus whereas the vaccinated group remained re-excreting very low levels of the genotype XIII virus.
Study conclusions
The results of this challenge study demonstrated that Vectormune ND induced very strong clinical protection against the genotype XIII NDV strain from India with significant reduction of the re-excretion of the challenge virus.
From the practical point of view, associating strict biosecurity procedures with a vaccination program including Vectormune ND vaccination in the hatchery (either at day-old, or by in-ovo route) provides the necessary protection for broilers. Additionally, thanks to its long duration of immunity (Palya et al., 2014) due to HVT persistence in the bird, this vaccine becomes a very convenient solution for long-lived birds as well.
As it was demonstrated by Tatár-Kis and co-workers, Vectormune ND reduces the transmission of Newcastle Disease virus in commercial broiler chickens (Tatár-Kis et al., 2020) (R<1). Likewise, the strong reduction of the genotype XIII shedding shown in this study may contribute to contain the spread of this virus to neighboring flocks or nearby farms. It can be regarded as an attractive option for poultry producers in India and nearby countries.
| References | ||||
|---|---|---|---|---|
| 1. Ballági-Pordany A., Wehmann E., Herczeg J., Belak S., Lomniczi B. | ||||
| (1996) | Identification and grouping of Newcastle disease virus strains by restriction site analysis of a region from the F gene.. Arch. Virol | 1996;141:243–261. | ||
| 2. Diel, D. G., da Silva, L. H., Liu, H., Wang, Z., Miller, P. J., & Afonso, C. L. | ||||
| (2012) | Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes.. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. | 12(8), 1770–1779. | ||
| 3. Dimitrov, K. M., Abolnik, C., Afonso, C. L., Albina, E., Bahl, J., Berg, M., Briand, F. X., Brown, I. H., Choi, K. S., Chvala, I., Diel, D. G., Durr, P. A., Ferreira, H. L., Fusaro, A., Gil, P., Goujgoulova, G. V., Grund, C., Hicks, J. T., Joannis, T. M., Torchetti, M. K., … Wong, F. Y. K. | ||||
| (2019) | Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus.. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. | 74, 103917. | ||
| 4. Gowthaman, V., Singh, S. D., Dhama, K., Ramakrishnan, M. A., Malik, Y. P. S., Gopala Krishna Murthy, T. R., Chitra, R., & Munir, M. | ||||
| (2019) | Co-infection of Newcastle disease virus genotype XIII with low pathogenic avian influenza exacerbates clinical outcome of Newcastle disease in vaccinated layer poultry flocks.. Virus disease | 30(3), 441–452. | ||
| 5. Herczeg J., Wehmann E., Bragg R.R., Dias P.M.T., Hadjiev G., Werner O., Lomniczi B. | ||||
| (1999) | Two novel genetic groups (VIIb and VIII) responsible for recent Newcastle disease outbreaks in southern Africa, one (VIIb) of which reached southern Europe.. Arch. Virol. | 1999;144:2087–2099. | ||
| 6. Khorajiya, J.H., Mathakiya, R.A., Joshi, B.P., Prajapati, K.S., Acharya, A., & Rajpura, R.M. | ||||
| (2015) | Isolation, identification, biological characterization and patho-epidemiology of genotype-XIII Newcastle disease virus outbreak in commercial vaccinated broiler farms.. Indian journal of poultry science, | 50, 132-137. | ||
| 7. Lomniczi B., Wehmann E., Herczeg J., Ballagi-Pordany A., Kaleta E.F., Werner O., Meulemans G., Jorgensen P.H., Mante A.P., Gielkens A.L., Capua I., Damoser J. | ||||
| (1998) | Newcastle disease outbreaks in recent years in western Europe were caused by an old (VI) and a novel genotype (VII). Arch. Virol. | 1998;143:49–64. | ||
| 8. Nath, B., & Kumar, S. | ||||
| (2017) | Emerging variant of genotype XIII Newcastle disease virus from Northeast India.. Acta tropica, | 172, 64–69. | ||
| 9. Nooruzzaman, M., Mumu, T. T., Kabiraj, C. K., Hasnat, A., Rahman, M. M., Chowdhury, E. H., Dimitrov, K. M., & Islam, M. R. | ||||
| (2021) | Genetic and biological characterization of Newcastle disease viruses circulating in Bangladesh during 2010-2017: further genetic diversification of class II genotype XIII in Southcentral Asia.. The Journal of general virology, | 102(3), 10.1099/jgv.0.001554. | ||
| 10. Palya, V., Tatár-Kis, T., Mató, T., Felföldi, B., Kovács, E., & Gardin, Y. | ||||
| (2014) | Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers.. Veterinary immunology and immunopathology, | 158(1-2), 105–115. | ||
| 11. Paniago, M., Gardin, Y., Palya, V., Cazaban, C., Lozano F., Tatár-Kis, T., Mató, T. Kiss, I. | ||||
| (2016) | Assessment of the protection induced by a vector rHVT-F vaccine against different genotypes of Newcastle Disease virus. | In Proceedings of the 65th Western Poultry Disease Conference (WPDC), p. 197-199. Vancouver, BC, Canada, April 24th – 27th, 2016. | ||
| 12. Russell P.H., Alexander D.J. | ||||
| (1983) | Antigenic variation of Newcastle disease virus strains detected by monoclonal antibodies.. Arch. Virol. | 1983;75:243–253. | ||
| 13. Tatár-Kis, T., Fischer, E. A. J., Cazaban, C., Walkó-Kovács, E., Homonnay, Z. G., Velkers, F. C., Palya, V., & Stegeman, J. A. | ||||
| (2020) | A Herpesvirus of Turkey-Based Vector Vaccine Reduces Transmission of Newcastle Disease Virus in Commercial Broiler Chickens with Maternally Derived Antibodies.. Vaccines | 8(4), 614. |









