Avian influenza: An in-depth look at a distressing disease
David Swayne explains how the avian influenza virus functions and why recent outbreaks have been so hard to controlAvian influenza (AI) is a highly contagious viral disease that spreads naturally among wild aquatic birds worldwide. Commonly called ‘bird flu’, avian influenza can infect domestic poultry and other bird and animal species. Domestic flocks are especially vulnerable during seasonal migrations when wild birds move from one region to another. In a keynote address at the International Egg Commission annual conference last year, veterinarian and global avian influenza expert David Swayne illustrated how the avian influenza virus functions, and explained why recent outbreaks have been so difficult to control.
Low-pathogenic vs high-pathogenic avian influenza
Avian influenza viruses can be categorised into two sub-groups: low pathogenic viruses (LPAIV) and highly pathogenic viruses (HPAIV). Low pathogenic viruses usually only cause mild symptoms, while highly pathogenic viruses can be extremely deadly.
Typically, low pathogenic viruses grow inside the respiratory tract where they cause respiratory infection in birds. Infections usually result in nasal or eye discharge. In active laying hens, low-pathogenic infections may also impact egg production and quality as the layer’s oviduct undergoes involution. The most widespread low-pathogenic avian influenza strain at the moment is the H9N2 strain.
But it’s not the low-path viruses that are wreaking havoc on flocks around the world; it’s the highly pathogenic viruses, specifically H5N1.
When poultry contract highly pathogenic avian influenza the disease manifests in a number of ways. In some cases, birds’ heads appear swollen, especially around the eyes. In others, the comb and waddle may turn black. As the virus attacks the blood vessels, it produces thrombi and emboli that plug vessels and stop oxygen flow, killing tissue in the process. Highly pathogenic viruses can also cause hemorrhaging, especially under the skin.
“[H5N1] causes severe disease that affects almost all cells in the body,” Swayne explained.
How avian influenza evolves and spreads
Research going back as far as the 1960s shows that low-path viruses live in aquatic birds, such as ducks and geese, without causing any detrimental effects. Over time, however, and with increased exposure, viruses infect commercial and backyard poultry, including broilers, layers and turkeys.
“Historically, migratory aquatic birds have been the reservoir for all low-pathogenic avian influenza viruses,” said Swayne. “Wild waterfowl have not been the reservoir for high-path AI viruses, but that has changed.”
The virus’s transition from low-path to high-path isn’t immediate. It has to go through a process before it adapts to the new species. Once it goes through the adaptation process, the viruses have the ability to circulate freely in terrestrial poultry.
“They usually do not go back into the wild birds,” Swayne explained. “The wild birds are involved in the beginning as a low-path virus and then it moves into domestic poultry. But once it adapts, it stays within domestic poultry.”
Furthermore, some strains – the H5s and H7s, for example – mutate in the hemagglutinin protein. That mutation is what makes it a high-pathogenic virus.
How does this mutation take place? Avian influenza is a very small virus with just two protein projections on its surface. One is a hemagglutinin and the other is a neuraminidase. There are 16 hemagglutinin subtypes (H1–H16) that keep changing over time as the virus mutates.
Swayne said one of the reasons the virus is so complex is that its genetic code is contained in eight separate gene segments. When two different influenza viruses come together in a single cell, for instance, they produce a hybrid virus.
“It gets the best gene segments in there to make that virus.,” explained Swayne. “Therefore, it may change its hosting adaptability.”
What countries do when avian influenza strikes
The vast majority of virus strains are what experts call ‘emergent viruses.’ Most countries will try to eradicate emergent viruses immediately, Swayne said. However, a few of the high-path strains are now entrenched, meaning they’re present in domestic poultry and spread around by birds on small operations and in live markets. There are even some strains entrenched in wild bird populations as well.
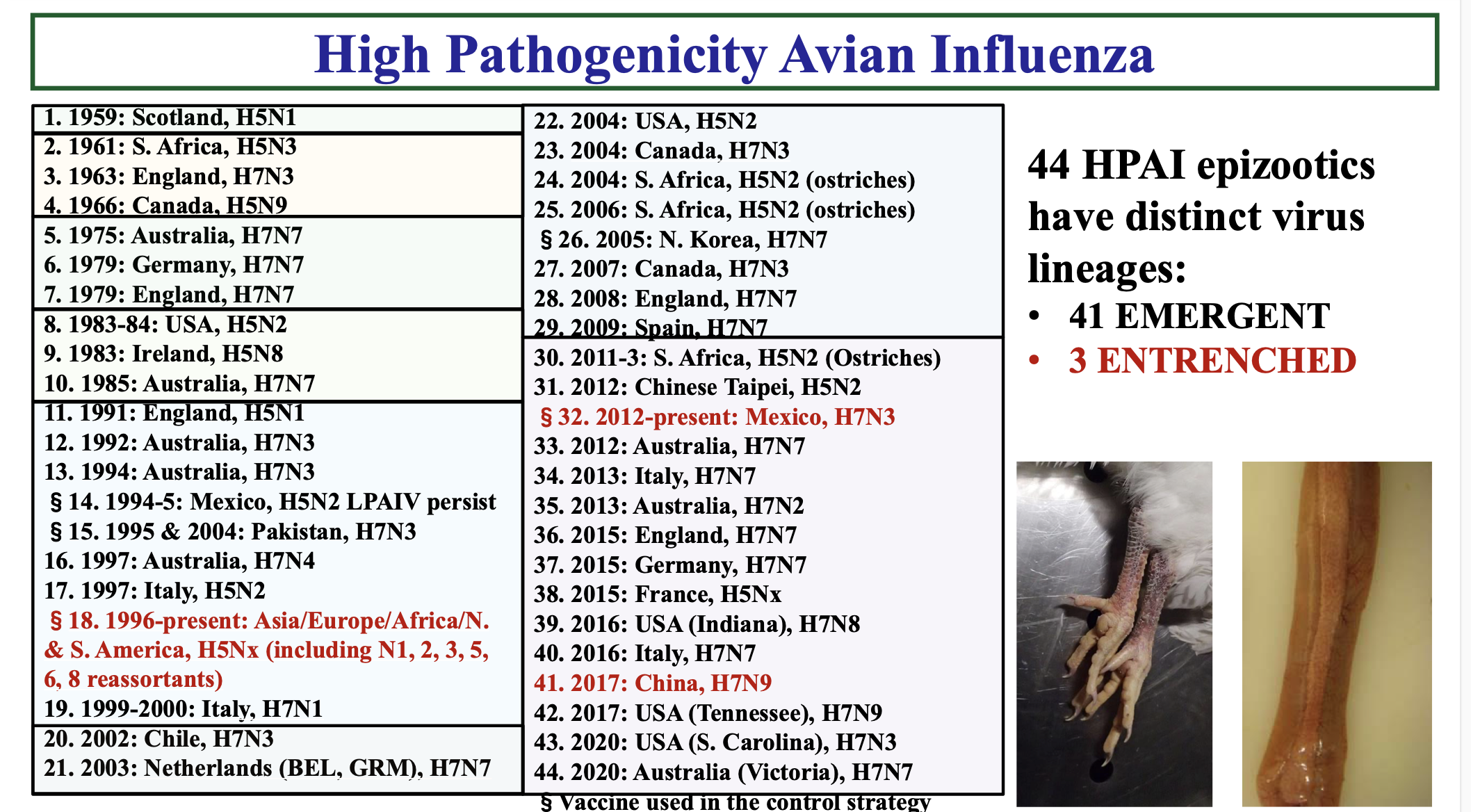
Between 1959 and 2020, officials recorded 44 highly pathogenic avian influenza epizootics with distinct virus lineages. Of those, 41 have been categorised as ‘emergent’. This means that as they appeared, governments acted quickly and successfully eradicated it. Of those, three are entrenched and still circulating in poultry in a number of countries. The current H5N1 strain, for example, has affected poultry in 114 countries, of which 72 were hit in just the last three years.
Case Study: A closer look at the 2020 outbreak in North and South Carolina
In March 2020, an emergent, high-path avian influenza virus surfaced in the United States. Over the next month, the virus hit 11 commercial turkey breeders and one meat operations. Impacting operations in North Carolina and South Carolina, the outbreaks were isolated to just three very small counties.
Further research revealed that the outbreak resulted from a single introduction of a low-pathogenic H7N3 virus to a single turkey operation in North Carolina. From there, it spread to several other farms, and on one of those farms it mutated into a highly-pathogenic virus.
All 12 turkey operations were quickly depopulated, and within two months, the pathogen was completely eradicated.
The case of the Goose Guangdong Eurasian virus
Virus strains – such as the H5 and H7 strains – are completely unique, genetically speaking. Within the H5 category, individual strains are distinct, usually differentiated by continent.
One virus, of Eurasian lineage, has seen incredible change since it first surfaced in 1996, according to Swayne. Researchers refer to this strain as the goose/Guangdong/1/96-lineage H5 HPAI.
“That’s the virus we’re fighting today,” said Swayne. “Since 1996 this virus has been around, and we’re still fighting it today. It’s continued to mutate in the hemagglutinin and reassort all those other genes.”
What’s unique about the Goose Guangdong virus is that throughout its biological history the virus has continually interacted between domestic ducks and terrestrial poultry.
“That virus was continuing to contain the ability to infect both waterfowl and ducks, as well as chickens and turkeys,” said Swayne. “Because of that waterfowl connection, that virus could easily move back and forth between wild waterfowl into domestic ducks.”
“In this way, our Achilles heel is our domestic ducks,” he added. “They are the most susceptible of all our poultry species to this high-path AI virus.”
In one study using an H5N1 strain from Mongolia, the virus was so virulent it killed all of the two-week old mallards involved in the study in just five days. When that same strain was inserted in juvenile mallards, the ducks showed no sign of illness and none died. However, the same ducks that showed no sign of illness were capable of shedding the virus through their faeces and through respiratory secretions for up to 14 days following infection.
“This means they could be infected asymptomatically as juvenile ducks, and they could move and shed that virus for periods of time into the environment and be a source of movement and transmission,” explained Swayne.
What’s clear from the studies that have been conducted is that the Goose Guangdong virus has wreaked more havoc than all of the other 43 virus strains combined. It is so virulent that it sometimes takes as little as 100 particles to infect a host. Today, over 114 countries have been impacted by this particular lineage. It is the largest outbreak of a single high-path avian influenza strain to date.
“The establishment of this virus in some wild birds is what we call endemic status,” he said. “It’s there, it’s going to be there for a while, and it continues to be a source of infection in the geographic area.”
Highly-pathogenic avian influenza cases have remained high since 2002. There have been five different peaks based on the number of reported cases, as well as the number of affected birds. Most of those have just one virus lineage – the Goose Guangdong lineage – but within that lineage there have been spikes where the virus has changed by mutation or reassortment. The result has been devastating. Since 2005, 38,658 premises have been affected, killing over 31 million poultry. Additionally, 483 million birds have been culled or disposed of in an attempt to control the virus.
Since 2020, 7,500 outbreaks have been reported. The outbreaks have caused over 14 million poultry deaths, and an additional 254 million birds have been culled.
The Eurasian H5N8 outbreaks
The wave of Eurasian H5N8 outbreaks began in the fall of 2020 in central Asia. As the virus moved, an H5N1 reassortant replaced H5N8, causing infection in diverse wild bird species that resulted in both illness and death.
The virus spread via migratory birds across areas on five continents, including Asia, Europe, Africa, and North and South America. There has even been spillover to predatory and scavenger species of wild birds, and into poultry.
According to Swayne, data reveals that poultry infections were the result of direct spread by wild aquatic birds and indirect spread via contaminated faeces in and around poultry barns. In some countries, the virus was spread from farm to farm via human activity. Spill-over cases have emerged in wild carnivorous terrestrial and sea animals, and even in humans from direct poultry exposure.
Understanding virus virility
The nature and size of virus particles make it keeping disease at bay a challenging task. Each gram of faeces from a contaminated bird contains about 10 million virus particles. Respiratory secretions contain about 100 million virus particles.
“That’s a lot of virus in a very small amount of material,” said Swayne. “It makes you understand why biosecurity is so critical.”
It takes fewer virus particles to infect a flock than one might think. Studies show, for instance, that it takes just 80–1,000 virus particles to produce an infection in domestic ducks. In turkeys, 10,000 and 16,000 virus particles are enough to infect a flock with a low-path virus.
Chickens are much more resistant than ducks and turkeys; typically, it takes it takes 15 to 200 million low-path particles to produce an infection. However, when high-path viruses are present, it takes as little as 1,000 and 50,000 particles to produce an infection.
“If we look at big outbreaks that spread like wildfire, it took a very, very small amount – as little as 16 virus particles to about 1,000,” said Swayne.
During the 2014–15 outbreaks, just 500,000 particles produced infections in chickens, and about 100,000 particles produced infections in turkeys.
“That explains why in the Pacific Flyway, those first cases that we saw were only in backyard flocks that had direct exposure to a lot of virus,” Swayne explained. “But the current virus, when it came, it was already adapted for chickens and turkeys.”
“That virus came in ready to infect our poultry,” he added.
Biosecurity as a frontline defence
Biosecurity remains poultry producers’ strongest defence. The goal of any biosecurity program is to keep an invisible pathogen away from poultry and that’s done through a series of best management practices.
For the best chance of success, each poultry farm should have its own comprehensive biosecurity plan that is written down and clearly communicated to everyone who enters the farm premises. Biosecurity plans should be regularly audited in order to find and correct any weak links.
On poultry farms, the barn door should be considered the ‘stopping point’ for virus entry, and the yard around the bar should always be considered contaminated.
Beyond the barn, a strong avian influenza response plan includes detection, quarantine, depopulation and virus elimination. Unfortunately, viruses will persist regardless of how tight a biosecurity plan is, but that’s no reason to slack, Swayne said.
“We have to understand that biosecurity reduces the risk, but it doesn’t eliminate it,” he concluded. “The practice of biosecurity takes dedication, consistency and an all-in approach.”