



DNA Vaccines and Future of Disease Control
UK - It often makes sense to cut out the middleman. Be it a personal choice or business decision, cutting out unnecessary steps can save time, energy, and be a better tactical and economical bet.And what goes for lifestyle also holds true for life. In biology, cutting out the middleman may yet pay off in the development of DNA vaccines to protect against animal diseases.

Using DNA vaccines, rather than inoculating a person or animal with vaccines based on real infectious agents offer many benefits, including avoiding the risk of reinfection, and the expense of cultivating and handling deadly viruses and bacteria.
But removing middlemen from the equation is not always easy. DNA vaccines have yet to transform the market as many scientists expected years ago, despite the potential to enable healthy living more simply and safely than conventional vaccines. BBSRC-funded researchers are thus exploring the technology to see how it could be used to protect cows and chickens from diseases, mixing theory and practical application to answer modern food security and animal welfare challenges.
The next generation
Vaccination is arguably the most effective defence ever deployed to fight disease. Along with access to clean water and the development of drugs to fight infections, vaccination strategies have saved billions of animals and people from death, sickness and hardship.
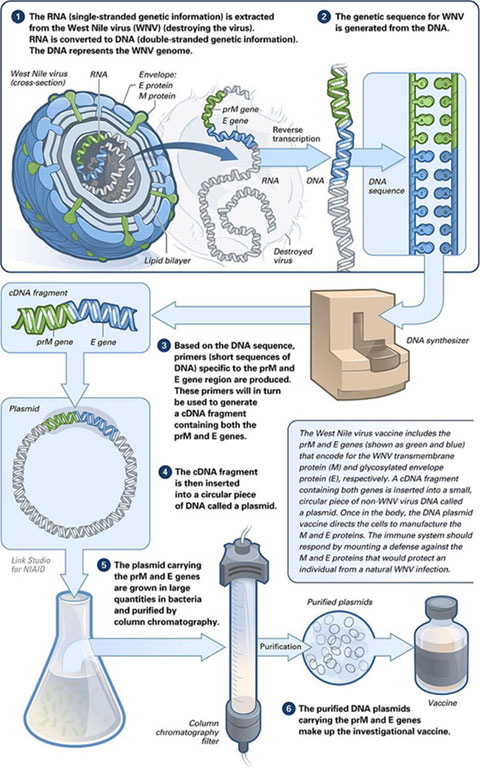
Image: National Institute of Allergy and Infectious Diseases
But using vaccines is not without problems. Deploying live (but weakened 'attenuated') viruses can sometimes result in the virus mutating and reverting back to its harmful ways, spreading a new strain of the disease.
"DNA vaccines can't start the disease," says Dr Paul Barnett of the Institute of Animal Health (IAH) in Pirbright, UK, which receives strategic funding from BBSRC. Barnett has demonstrated for the first time that cattle can be clinically protected against FMD virus by tactical delivery of a DNA vaccine, albeit with the right accompaniments (ref 1).
DNA vaccines can circumvent the general reinfection danger because they contain only DNA and not infectious agents. So rather than introducing a virus or bacteria (antigen, or part of) to provoke a protective immune response in the form of host antibodies, DNA vaccines only carry the agent's genetic code. The result is the same – antibodies are produced that remain in the body to guard against infection; the middleman infectious agent remains in a test tube out of harm's way.
This approach offers other advantages: DNA vaccines are stable at a wider range of temperatures, reducing the need for costly and logistically challenging cold-delivery chains that can prevent treatments getting to where they are most needed, particularly in the developing world. DNA vaccines are easier to manipulate than many conventional vaccine elements, and genomic technology can make bespoke sequences (which are delivered as DNA loops called plasmids) quickly to tackle rapidly emerging threats, such as food poisoning outbreaks, or local variants of established diseases.
And culturing infectious agents for vaccines requires expensive quarantine facilities that can never be 100 per cent failsafe – accidental (or even malicious) release into the environment is always a possibility.
The undiscovered immunity
But biological discovery, as life, is not always a smooth and straightforward path. By the 1990s, DNA vaccines were aimed at more than 30 animal diseases, yet despite more than two decades of research there are only three licensed vaccines – for West Nile virus in horses, haematopoietic necrosis virus in salmon, and melanoma in dogs [Expert Reviews Vaccines, in press].
This is because DNA vaccines lead to a wider array of immune responses than conventional immunisation techniques because the precise metabolic machinery that leads to a favourable immune response is not fully understood. Hence, researchers have grappled with various factors such as site and method of injection to improve host immunity, coupled with a repertoire of chemical extras (adjuvants) that are used with vaccines of all types to elicit more favourable cell responses.

For instance, there are three main methods of applying DNA vaccines: needle inoculation; electroporation, which is using a current to create pores in cell membranes for DNA to pass through; and use of a 'gene gun', a device that propels DNA-coated gold particles directly into live cells. All three methods elicit different cellular reactions because they may stimulate, or bypass, important immunological pathways that lead to immunity.
Then there is the site of injection: what's good on one animal, such as the ears of a pig, doesn't work for other livestock such as sheep or cows.
And yet another way to tinker with the efficacy of the DNA vaccine is to add other biological ingredients into the mix. These take the form of short sequences of DNA or protein (peptides) called prime boosts which can increase antibody numbers, as well as their activity and persistence. Recombinant (from engineered bacteria) proteins or viruses can also induce better immune responses when used in conjunction with DNA vaccines, activating new classes of immune cells into the fight. To tackle foot-and-mouth disease virus (FMDV), Barnet used a prime-protein boost strategy. The prime is DNA coding for the full capsid (protein shell) structure of the virus. The protein boost is essentially the same antigen component used in the traditional FMD vaccine – chemically inactivated FMD virus.
So aren't the benefits lost if the conventional vaccine is also used? Not so says Dr Barnett. "There are reasons why we went for this approach. Firstly, in pigs it was shown that this prime-protein boost regime gave a considerably higher antibody response than is normally seen by conventional vaccine," he says. "Moreover, the response was more cross-reactive across different serotypes [strains of virus]."
Obtaining a one-size fits all vaccine that works against serotypes is, as Barnett puts it, "a holy grail in FMD vaccine research." He adds that if the immunity was enhanced by a DNA prime-protein boost to the extent that you stopped cattle getting persistently infected this approach was even more valuable. "However, unfortunately it seems the merits of this approach in pigs were not substantiated in cattle."
Barnett also experimented with various adjuvants to make his DNA vaccines work against FMDV. One of the most effective is an extra DNA plasmid that codes for the cytokine, bovine granulocyte-macrophage colony-stimulating factor, or GM-CSF. This is useful because cytokines are a large class of protein molecules used extensively in intercellular communication, and four of six previous studies using cytokines with DNA vaccines have reported full protection in pigs (ref 1).
"GM-CSF is a cytokine and is a key part of the immune-inflammatory cascade, by which activation of a small number of macrophages can rapidly lead to an increase in their numbers, a process crucial for fighting infection," says Dr Barnett. "This function we think helps to enhance the vaccine's ability."
In addition, Dr Barnett found that electroporation was the best delivery strategy, protecting 75 per cent of animals after 91 days, compared to only 25 per cent of animals where electroporation was not used (ref 1).
First contact
Bird diseases are also a target for DNA vaccines. With 55Bn chickens produced worldwide in 2010 (ref 2), there is huge market potential for new avenues that can keep poultry free of disease, and without some of the problems associated with conventional approaches.
For instance, by just six weeks of age the average chicken has received between five and eight vaccinations; by 18 weeks this can be more than 12 live attenuated vaccines boosted with several inactivated shots to improve antibody levels in hens and offspring. So there is concern that intensive use of vaccines could be driving certain pathogens, notably Marek's disease herpesvirus (MDV) and infectious bursal disease virus (IBDV), to become more virulent (ref 3).

Professor Pete Kaiser at the Roslin Institute, Edinburgh, which also receives strategic funding from BBSRC, says DNA vaccination could provide several advantages over conventional vaccines. "Multi-component vaccines could reduce the number of vaccinations required during a bird's short life and the incorporation of immunostimulatory molecules could direct and enhance protective immune responses," he says. "Evoking more appropriate immune responses could avoid the risk of driving some pathogens to increasing virulence, as in the situation with MDV and IBDV."
One looming problem is that, at least in the US, vaccines are delivered in ovo (in the egg) and into the amniotic fluid that surrounds the yolk by an automated injection system (Invoject) that can treat 50,000 eggs an hour. Unfortunately, Professor Kaisers says that although there have been a few trials delivering DNA vaccines in ovo, directly into the embryo, or into the allantois, [a sac-like structure primarily involved in nutrition and excretion] no-one has experimentally delivered DNA vaccines into the amniotic fluid.
"Our unpublished work has shown that the amniotic fluid has DNase activity," Professor Kaiser warns. This is not ideal because the DNase enzyme will degrade the DNA vaccine, but Professor Kaiser suggests this could be circumvented by delivering the DNA in cationic liposomes – structures made of positively charged lipids which react well with negatively charged DNA and cell membranes. "The simple fact is that more research needs to be done in this area," says Kaiser.
At present, recombinant fowlpox virus vaccines are used in the poultry industry, but DNA vaccines are some years away. In fact, Kaiser thinks that subunit vaccines are the way to go for the poultry industry. These deliver proteins, or particles of viruses, bacteria or protozoa, rather than the agent itself. For DNA vaccines to become a commercial reality in the poultry industry, Professor Kaiser identifies three main challenges. "Identifying the correct antigens, adjuvants and delivery routes; then getting protection levels to those already given by live, attenuated vaccines. And he cites one last, ever-present consideration: cost.
The final frontier
DNA vaccines for cows and chickens may be some way from commercial reality, but their potential benefits – ease of making DNA, reactivity, thermostability, theoretical low cost, and proven effectiveness in other animals – means that further research will continue.
And there are other benefits, specific to certain animals and diseases. For instance, limitations of the current FMD vaccine include a lack of induction of sterile immunity; this is where in persistently infected animals, replicating FMD virus can be found in the samples of oropharyngeal fluid taken from the back of the throat, in either vaccinated or non-vaccinated animals, that have been exposed to the virus.
"The current FMD vaccine prevents clinical signs of the disease but doesn't stop this sub-clinical replication going on in this area," says Dr Barnett. "The fact that replicating virus can be isolated from these animals has always been a cause of concern, particularly to other countries which want to trade in animals and animal products."
This means that vaccinated and non-vaccinated animals cannot be distinguished, which can hamper control strategies. It also restricts the movement of animals, which is a major economic consideration at local, regional and global levels. DNA vaccines could, in theory, see an end to this problem.
Development of DNA vaccines is still a relatively young field, compared to the first FMD vaccine which was developed in Germany in 1937 for example. But in the age of genomics where DNA can be sequenced and created more quickly, accurately, and cheaper than ever before, and where safety and handling live pathogens will always be fraught with risk and difficulty, further research on DNA vaccines is surely a worthwhile pursuit when addressing modern food security, animal health and perhaps even human healthcare challenges.
References
- A DNA vaccination regime including protein boost and electroporation protects cattle against foot-and-mouth disease (external link)
- FAO Stat (external link)
- DNA vaccines for poultry: the jump from theory to practice (external link)








