IBV Variants in Poultry: Aiming for Cross-Protection
GLOBAL - Avian infectious bronchitis is an upper-respiratory disease in chickens. Highly contagious and thus worldwide in distribution, it is characterized by sneezing, gasping, coughing, watery eyes, tracheal rales and, sometimes, swollen sinuses.When the disease is complicated by secondary bacterial infections, it can result in airsaculitis. Layers and breeder birds in lay can experience a drop in production; wrinkled and misshapen eggs are often observed, writes Mark W. Jackwood, PhD in Poultry Health Today. Some types of the virus cause lesions in the kidneys. They are known as nephropathogenic strains and can result in significant mortality.
Infectious bronchitis is extremely difficult to manage because different types of the virus causing the disease do not cross-protect. Currently, the best strategy is the use of modified live infectious bronchitis virus (IBV) vaccines. However, there are a number of different IBV types circulating in commercial chickens, and new IBV types are continuously arising because the virus is able to constantly and rapidly change.
Obviously, it’s important to identify the IBV type responsible for disease, but even when the cause of an outbreak is determined, IBV management may be hindered by the limited number of different commercial IBV vaccine types available.
Quick history
Infectious bronchitis was first recognized around 1930, and for over 15 years, only one serotype, designated Mass because it was isolated in Massachusetts, was known. In 1956, a virus was isolated in Connecticut by Jungherr et al[1] that could not be neutralized by antisera against the Mass virus, and thus a second serotype, designated Conn, was identified.
For many years, this type of characterization was made using the virus neutralization (VN) test to identify new IBV serotypes. However, few new viruses were identified because the test was labor intensive and difficult to perform.
In the 1990s, molecular IBV diagnostic testing was developed, and it revolutionized the way we diagnose and control infectious bronchitis. Using molecular tools, we now know that there are many different IBV types all over the world.[2] In addition, molecular analysis of IBV has provided and continues to provide important data on the molecular changes associated with this virus.
Generation of new variants
Viruses in general contain either a DNA or RNA genome. The DNA viruses are relatively stable. In contrast, RNA viruses are constantly undergoing genetic change because of the replication mechanism they use. The IBV genome — the set of genes present in the virus — consists of a very long, single RNA strand, and the rate of change is extremely high. Genetic changes in IBV follow typical Darwinian evolution that can be measured in real time.
There are two mechanisms involved in IBV genetic change. The first is the generation of mutations that accumulate over time, resulting in genetic drift. This occurs because the polymerase enzyme makes mistakes as it replicates the viral RNA genome to create a new virus particle.
Not only does the polymerase enzyme make mistakes at a high rate, it has limited ability to go back and fix those mistakes, resulting in rapid evolution of the virus. When the mistakes introduced into the genome provide a selective advantage for the virus, that “genetic variant” becomes the new virus emerging to cause disease.
Viral molecular evolution is constantly occurring in IBV and was documented in 1998 when the DE/DE072/92 virus (originally isolated in 1992) circulating in chickens evolved into the GA98/0470/98 type virus.[3] Essentially, changes in the viral genome that accumulated over a 6-year period of time led to the emergence of GA98/0470/98 in 1998, which was genetically 93% similar and serologically distinct from DE/DE072/92 (Figure 1).
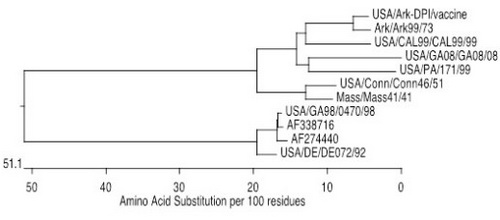
Figure 1. Phylogenetic tree for IBV S1 amino acid sequences showing the relationships between different isolates. Numbers at each node of the tree represent percent similarity between the sequences. Viruses AF27440 and AF338716 are sister viruses showing the progression of evolution from DE/DE072/92 to GA98/0470/98.
The second mechanism introducing change in the genome of IBV is called recombination. Recombination can occur when two different IBV types infect the same cell. During virus replication, the polymerase enzyme begins copying the genome of one virus then switches to the other virus, creating an offspring virus containing portions of each parent’s genome. This process results in a relatively quick change in the makeup of the viral genome and is called genetic shift.
Although genetic shift can result in rapid and significant changes, it is often inconsequential, for example, when two vaccine viruses recombine with an outcome of a virus that is still attenuated for chickens.
Only when recombination occurs in specific genes involved in pathogenicity and/or immunogenicity can a new virus emerge to cause disease. This is a relatively rare event, but it can happen as evidenced by a recombination event between IBV and an as-yet-unidentified coronavirus, resulting in the emergence of turkey coronavirus, a cause of enteric disease in poults.[4] Not only did the new virus emerge in a different species — from chickens to turkeys — its tissue tropism also changed from the respiratory to enteric tract.
Serotype, genotype, protectotype and variant
When IBV type is defined using neutralizing antibodies in the laboratory VN test, it is referred to as a serotype, and it is generally accepted that viruses within the same serotype will cross-protect in chickens. When the type of IBV is defined by the sequence of the viral genome, it is referred to as a genetic type or genotype. When chickens immunized with one IBV type are protected against another IBV type used as a challenge virus, they are called protectotypes because cross-protection is actually measured in chickens.
A variant IBV is a virus that has changed to the point where it can be identified as different or unique from other known IBV types. A variant virus can be either a serotype variant or a genetic variant, and the term implies that little or no cross-protection occurs with other known IBV types.
Changes in spike matter
The spike glycoprotein, located on the surface of the virus (Figure 2), is involved in attachment and entry into the cells of the upper-respiratory tract, and it induces the production of neutralizing antibodies. When birds are vaccinated, it is those neutralizing antibodies that bind to the spike glycoprotein and prevent infection.
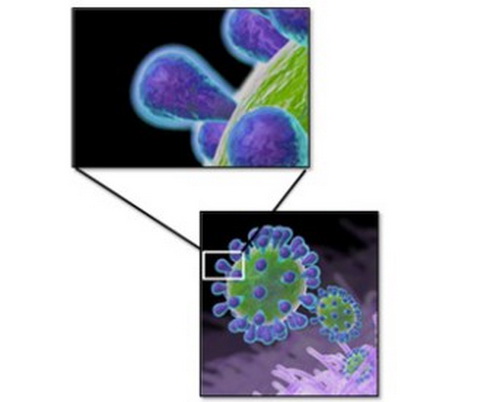
Figure 2. Spikes on the surface of IBV induce neutralizing antibodies and are different for each serotype.
Changes in the gene that codes for the spike glycoprotein can result in different amino acids, making up specific areas where the neutralizing antibodies bind to the spike (e.g., a variant virus); the result is a new virus that can infect and cause disease. Thus, changes in the spike glycoprotein are meaningful when evaluating cross-protection, and this is why the spike glycoprotein sequence is used to genetically type IBV isolates. There is a good correlation between closely related spike sequences and cross-protection. Other regions of the viral genome cannot be used to infer protection between two IBVs.
Computer programs that align the sequences of two or more spike glycoproteins can be used to determine the similarities or differences between viruses. Sequence alignments are very difficult to read, so the data is usually reported in a percent identity (similarity)/divergence (difference) table (Table 1) or graphically in a phylogenetic tree (Figure 1).
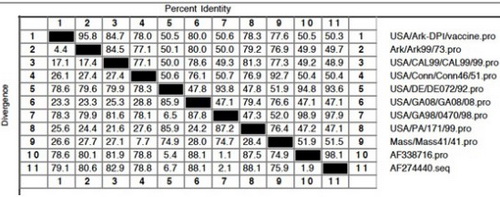
Table 1. Percent identity (similarity) and divergence (difference) for IBV S1 amino acid sequences
The length of the branches — the horizontal lines — on the phylogenetic tree represents the percent similarity or difference between the viruses and can be used to make reasonable predictions regarding cross-protection. As a general rule, viruses that have spike glycoprotein amino sequences that are 95% similar or higher will provide a good level of cross-protection, and viruses between 88% and 95% often provide partial protection. However, caution should be used when making these general statements because exceptions do exist. Some viruses with similarities in the 95%+ range provide little cross-protection, whereas other viruses with much lower similarities in spike provide surprisingly good cross-protection.
Cross-protection and managing variant viruses
Antibodies in the upper-respiratory tract bind to the virus and prevent infection, and as indicated above, those neutralizing antibodies specifically bind to the spike glycoprotein. It is generally accepted that different types of the virus do not cross-protect, but there are some gray areas.
We know that the relationship between two viruses matters: Closely related viruses provide more cross-protection against each other than distantly related viruses. However, distantly related viruses can provide some cross-protection. This is likely due to the production of antibodies that bind to regions adjacent to areas where neutralizing antibodies actually bind (Figure 3). The position and strength of antibody binding to other areas on spike will influence the outcome of neutralization.
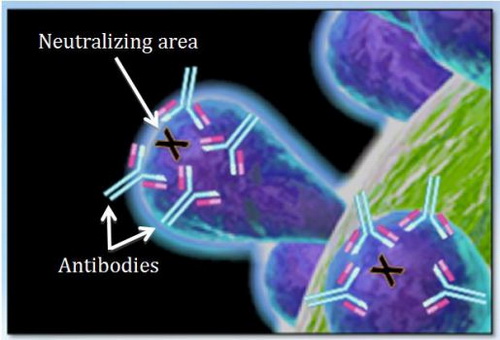
Figure 3. Antibodies binding to conserved regions around neutralizing areas on the spike glycoprotein.
A lot of research has been conducted on the protective ability of different IBV vaccines or combinations of IBV vaccine types. In general, using more than one type of IBV in a vaccine program will provide broader protection than the use of just one type of IBV vaccine.
It has also been discovered that some IBV vaccine combinations, such as a Mass-type vaccine followed by a 793B-type vaccine, are particularly good at inducing antibodies that will cross-protect or partially protect against a number of different IBV types.[5] However, no specific vaccine or combination of vaccines will protect against all IBV types, and the only sure way to determine if a vaccination program will work is to test it experimentally in chickens against the variant virus in question.
In summary, IBV is constantly undergoing Darwinian evolution as it replicates in the chicken, resulting in the emergence of new types of the virus. Changes in the spike glycoprotein do matter, with the general rule that the closer two viruses are related in the spike glycoprotein sequence, the more likely they are to cross-protect.
Finally, some IBV vaccine types are particularly good at inducing antibodies that will cross-react with more distant virus types, providing a broad range of protection, but each case is different and must be tested experimentally in chickens.
Mark W. Jackwood, PhD
Poultry Diagnostic and Research Center
Department of Population Health
College of Veterinary Medicine
University of Georgia
1Jungherr EI, et al. Immunologic differences in strains of infectious bronchitis virus. Proceedings of the 60th Annual Meeting U.S. Livestock Sanitary Association. 1956;203-209.
2Jackwood MW. Review of infectious bronchitis virus around the world. Avian Dis 2012;56:634-641.
3Lee CW, et al. Origin and evolution of Georgia 98 (GA98), a new serotype of avian infectious bronchitis virus. Virus Res 2001;80:33-39.
4Jackwood MW, et al. Emergence of a group 3 coronavirus through recombination. Virology 2010;398:98-108.
5Cook JKA, Orbell SJ, Woods MA, Huggins MB. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Path 1999;28:477-485.