Antibiotic-free and reduced antibiotic use in broiler production: Coccidiosis prevention
An Aviagen Focus SeriesIntroduction
The production of chickens raised without antibiotics (RWA) in the United States (US) has steadily grown during the last decade. The current production of RWA chickens is estimated to have surpassed 50% of the total chickens produced. This trend started in response to concerns raised by the World Health Organization (WHO) on the emergence of multi-antimicrobial resistant human pathogens. RWA production has been accelerated by demands made and pressure applied by consumer activists’ groups on regulatory agencies, grocery retailers, fast-food outlets, restaurant chains and poultry companies. In turn, large buyers of chicken products have demanded that broiler companies supplying them comply with these demands.
In some instances, marketing campaigns initiated by fast-food outlets, restaurant chains, or poultry companies themselves have resulted in the increased production of RWA chickens. As the RWA segment of poultry production continues to grow, veterinarians and production managers must practice more diverse management practices to prevent and control disease.
In the US, broiler chickens marketed under the NAE (no antibiotics ever), RWA or organic labels must be raised to market age without antibiotics, and all claims on how the chickens were raised must be substantiated. This requirement includes all antibiotics, even those that are not considered important in human medicine. In the US, anticoccidials belonging to the polyether-ionophore class, most commonly referred to as “ionophores,” are considered antibiotics by the Food & Drug Administration (FDA) and the United States Department of Agriculture (USDA). Therefore, even though ionophore anticoccidials are not medically important, their use in RWA poultry production is not permitted.
Prevention Without Antibiotics
Paralleling the increased production of RWA poultry, coccidiosis and necrotic enteritis (NE) have risen to the top of disease concerns among poultry veterinarians involved in broiler production. The removal of the ionophore anticoccidials and antibiotics with good anticlostridial activity means that the prevention and control of the most prevalent diseases of broilers must be achieved with fewer options. It is important to highlight that if birds become ill with a bacterial disease that can be appropriately treated with the judicious use of antibiotics, veterinarians must prescribe antibiotic treatment to ensure the birds’ health and welfare. In the case that this occurs, antibiotic-treated flocks will not be suitable for ABF products. Producers of RWA chickens/poultry must rely exclusively on the use of chemically synthesized anticoccidials, frequently referred to as “chemicals,” or live coccidiosis vaccines, or more than likely, a combination of the two.
Prevention with Chemically-Synthesized Anticoccidials
Except for organic producers, RWA production systems allow the use of synthetic anticoccidials for coccidiosis prevention. RWA producers can make use of this flexibility. Since intestinal lesions caused by coccidian parasites like Eimeria maxima are a well-known predisposing factor for NE, good coccidiosis control is essential. Chemically synthesized anticoccidials can be very effective, but with a few exceptions (e.g. nicarbazin, which has been successfully used since 1955), are more prone to developing resistance.
Although good coccidiosis control should help prevent NE cases, it is important to remember that, unlike the ionophore anticoccidials, synthetic anticoccidial medications or chemicals do not have anticlostridial activity and, therefore, broiler flocks should be closely monitored for the occurrence of NE.
When looking for successful long-term prevention of coccidiosis with chemically synthesized anticoccidials, a multitude of factors must be considered:
- Chemically synthesized anticoccidials have important differences in their spectrum of activity, mode of action, suppression of oocyst shedding, development of immunity, dose range, propensity to induce resistance, flexibility for use in any season, side effects and tendency to cause residues.
- Alternate use of anticoccidials with different modes of action require a carefully planned schedule that provides enough rest time between use periods to minimize the risk of developing resistance.
- Anticoccidial sensitivity tests (ASTs) repeatedly and routinely conducted can provide a valuable guide to timing and planning chemical anticoccidial use.
- Live coccidiosis vaccines must be considered a valuable rotation alternative to extend the efficacy of anticoccidials and minimize their risk of developing resistance.
Anticoccidial Sensitivity Testing
Chemically synthesized anticoccidials have been used for many decades, and with a few exceptions, are more prone to the development of resistance. Nevertheless, they are the only ones allowed in the production of RWA poultry. Table 1 shows some of the most common chemical anticoccidials approved by the FDA for use in chicken feed in the US, their year of initial approval and their current availability in the market.
Table 1. Chemically synthesized anticoccidials approved by the FDA for use in chicken feed.
|
Anticoccidial |
Year of Approval |
Available for Use |
|
|
Generic Name |
Trade Name |
||
|
Amprolium |
Amprol |
1960 |
Yes |
|
Amprolium + Ethopabate |
Amprol Plus |
1963 |
No |
|
Clopidol |
Coyden |
1968 |
Yes 1 |
|
Decoquinate |
Deccox |
1970 |
Yes 1 |
|
Diclazuril |
Clinacox |
1999 |
Yes 1 |
|
Halofuginone |
Stenorol |
1987 |
No |
|
Nicarbazin |
Nicarb |
1955 |
Yes |
|
Robenidine |
Robenz |
1972 |
Yes |
|
Zoalene |
Zoamix |
1960 |
Yes |
1 Availability has been variable.
Although molecular techniques have advanced a great deal in recent years, there is still no practical molecular test to easily identify resistance genes for the various anticoccidials in the three most prevalent coccidia species that infect broiler chickens (i.e. E. acervulina, E. maxima and E. tenella). Therefore, we must rely on the gold standard, AST, to monitor the efficacy and properly manage the development of resistance to anticoccidials. Since verification of sustained efficacy and early detection of resistance are of paramount importance in the management of anticoccidial programs involving chemical anticoccidials, ASTs should be an integral part of RWA programs.
General Observations of Synthetic Anticoccidials
Chemically synthesized anticoccidials were introduced much earlier (Nicarbazine, 1955) than the first ionophore anticoccidial (Monensin, 1971). Diclazuril, the most recently approved synthetic anticoccidial for use in broilers, was introduced more than 20 years ago. Even though it is thought that synthetic anticoccidials are very potent medications and tend to induce resistance very quickly, this is not true for all synthetic anticoccidials. Some, like nicarbazin, have been used successfully for decades, while others like zoalene have been re-introduced after a very long period (more than 20 years) of rest. No scientific studies have demonstrated how long the resting period must last to restore sensitivity once resistance has developed to a synthetic anticoccidial. However, recent experiences with anticoccidials like clopidol, decoquinate, and zoalene have shown that many years may be required for the parasites to regain sensitivity; this is another good reason for advocating rotations between synthetic anticoccidials and live coccidiosis vaccines. The Eimeria spp. included in live coccidiosis vaccines are susceptible to all anticoccidials. Scientific evidence has shown that coccidiosis vaccine use can, over time, aid in restoring sensitivity to anticoccidials by seeding broiler houses with sensitive strains of parasites that gradually inter-breed and displace the wild resistant strains. Of course, the duration of the regained sensitivity to anticoccidials is not the same as when the medications were originally introduced, presumably because when they are re-introduced, they can kill off the anticoccidials-sensitive vaccine strains of parasites and can permit over time the anticoccidials-resistant wild strains to repopulate the chicken houses.
Prevention with Coccidiosis Vaccines
In the US, the first live coccidiosis vaccine to prevent coccidiosis in chickens was introduced in 1955. Since then, several other coccidiosis vaccines have been introduced. Table 2 shows the list of live coccidiosis vaccines approved by the USDA Center for Veterinary Biologics (CVB) for chickens in the US.
Table 2. Live coccidiosis vaccines available for use in chickens in the United States 1.
|
Vaccine - Trade Name |
Bird Type |
Manufacturer |
|
Coccivac – B52 |
Broilers & roasters |
Merck |
|
Coccivac – D2 |
Layers & breeders |
Merck |
|
Immucox 3 |
Broilers & roasters |
CEVA |
|
Immucox 5 |
Layers & breeders |
CEVA |
|
Advent |
Broiler chickens only |
Huvepharma |
|
Inovocox EM1 |
Broiler chickens only |
Huvepharma |
|
Hatch-pack Cocci-III |
Broiler chickens only |
Boehringer-Ingelheim |
1 All vaccines for broilers contain at least E. acervulina, E. maxima and E. tenella, and all vaccines for breeders contain at least E. acervulina, E. brunetti, E. maxima, E. necatrix and E. tenella.
For years, live coccidiosis vaccines have been used in broiler breeder pullets, where long-lasting immunity is required to prevent outbreaks during production. In contrast, for many decades, coccidiosis control in global broiler chicken production has been successfully achieved primarily through the inclusion of anticoccidial medications in the feed. During the last decade, however, the production of RWA broilers in the US has increased significantly. Unlike other countries (i.e. the United Kingdom and the European Union), the ionophore class anticoccidials are considered antibiotics by the FDA and USDA. Therefore, using ionophores in RWA chicken production is not permitted, resulting in a significant increase in the use of live coccidiosis vaccines by broiler chicken producers.
General Observations of Coccidiosis Vaccines
Live coccidiosis vaccines are commonly administered by spray cabinet on the day of hatch or by in-ovo injection when eggs are transferred from the setter to the hatcher. Provided the vaccines are properly stored, mixed and administered, they can be effective. However, for the vaccine to induce active immunity, it has to infect and replicate several times in the intestines (Figure 1). In some cases, especially if the number of oocysts ingested is excessive, damage to the intestinal epithelium can occur and predispose chickens to secondary bacterial infections like those that cause NE. The dose per chick of a coccidiosis vaccine administered at the hatchery is calculated to prevent the ingestion of excessive amounts of oocysts that can lead to problems like NE. However, it is extremely difficult to get 100% coverage of the chicks during vaccination with the current vaccine administration methods. A chick that has not acquired a dose of vaccine at the hatchery is at risk for developing NE if and when it ingests an excessive amount of sporulated oocysts on the farm (by consuming oocyst-rich droppings from other fully vaccinated chicks). Unlike the controlled amount of vaccine sprayed on the chicks at the hatchery, the amount of sporulated oocysts present in the droppings from other chickens is uncontrolled and therefore, excessive amounts of oocysts can be ingested and increase the risk of NE outbreaks. The risk is greater if the amount of ingested sporulated oocysts is excessive for species like Eimeria maxima that infect the mid-intestine and are deep invaders. The prevention of coccidiosis and NE is intimately connected as intestinal lesions induced by coccidiosis (especially E. maxima), whether due to field challenge or use of live coccidiosis vaccine are well- known predisposing factors for clinical outbreaks of NE. Live non-attenuated and attenuated coccidiosis vaccines are available for use in chickens, including broilers and broiler breeder pullets. Live vaccines appear to produce better results in flocks raised on built-up litter; presumably, when sporulated oocysts left from previous flocks grown on the same litter are ingested, they may trigger trickle infections that aid in the development of immunity.
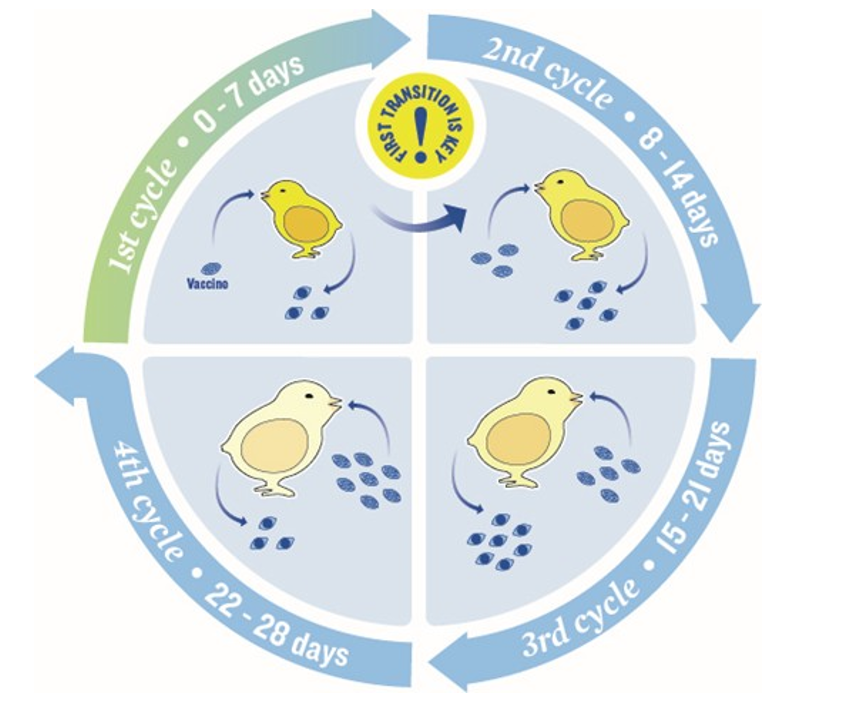
Dose/Administration-Related Problems
When a full dose of vaccine is administered individually to each broiler chick, all vaccines can induce a protective immune response to a challenge after 2-3 cycles of replication through the intestines have been completed. As previously mentioned, current methods of live coccidiosis vaccine administration at the hatchery do not produce 100% coverage and consumption or “take” of vaccine and broiler chicks that did not uptake the vaccine at the hatchery frequently succumb to NE in the field. Therefore, improving uptake of live coccidiosis vaccines is critical for success. Many strategies have been devised to boost oocyst ingestion and preening behavior to increase uptake of the vaccine, including:
- Adding dyes to the vaccine diluent to attract and encourage chicks to ingest vaccine droplets.
- Increasing the volume of vaccine sprayed to promote better coverage.
- Increasing chicks’ preening time in an area with high light intensity.
- Using colored gel diluents instead of water-based diluents to improve the detection and consumption of vaccine-containing droplets.
- Designing the spray cabinet to improve coverage.
In other cases, re-vaccination in the field (i.e. spraying another dose of the vaccine on the litter at chick placement) has been reported to improve coverage and protect broiler chicks that were missed at the hatchery.
Important Management Factors at the Farm
Once the broiler chicks have been placed, other factors need to be considered for adequate cycling and sporulation of oocysts and gradual development of active immunity through repeated infection. The most important factors are litter moisture, oxygen and temperature. In general, the oxygen and temperature requirements of the chickens are in the range required by the coccidian oocysts to sporulate, so the main concern is related to the amount of moisture present in the litter. If the litter is too dry, oocysts do not sporulate. Unsporulated oocysts are not infective, and therefore immunity development stalls. In contrast, an excessive litter moisture content is detrimental to oocyst sporulation. It has been reported that the highest desirable percentage of sporulation was achieved when the broiler house temperature was at 77°F or 25°C.
Depending on the Eimeria spp., with non-attenuated coccidiosis vaccines administered at the hatchery, the first shedding of oocysts occurs between 5 and 7 days, and the second one is approximately between 10 and 14 days. Therefore, in chicken houses where broiler chick brooding is confined to one half or one- third of the house, it is generally recommended to release the chicks from partial to whole-house brooding somewhere between the first and second shedding of oocysts (but not before ten days) or after the second shedding if the house conditions permit it.
A good way to confirm proper vaccine cycling is to look for the gross lesions of coccidiosis through a routine necropsy. Table 3 shows the expected incidence and severity of lesions as immunity develops for a live non-attenuated coccidiosis vaccine. Keep in mind that these are the lesions or oocyst counts that can be expected when the vaccine was properly administered on the day of hatch, the “take” was uniform, the house conditions ideal, and all the birds are cycling in synchrony. When any of the key factors previously discussed deviate from ideal conditions, lesions or counts may fall outside this range.
Table 3. Expected lesions and microscopic E. maxima scores after vaccination with a non-attenuated live coccidiosis vaccine 1.
|
E. acervulina |
E. maxima |
E. tenella |
|
|
Days |
18 – 21 days |
21 – 26 days |
20 – 23 days |
|
Peak Lesions |
Up to 15% +1 at 20 days |
No gross lesions |
<5% +1 |
|
Scores |
<10% +2 at 20 days |
30 – 50% at 21 – 26 days (1 – 10 oocysts/100X objective) |
0% +2 |
|
Declining or no lesions at 22 days |
< 10 – 20% at 21 – 26 days (10 – 40 oocysts/100X objective) |
Cecal cores: Rare (resolved infection) |
1 Merck Technical Services, 2020.
The coccidiosis lesions should appear at the expected time and be of the anticipated incidence and severity. The microscopic examination of mucosal scrapings from the mid-intestine should also show E. maxima oocyst counts in the expected range. In addition, fresh fecal samples can be randomly collected during the expected periods of oocyst shedding (e.g. every three days starting from 7 to 25 days of age) to conduct oocyst counts per gram of feces or OPGs. Although OPGs are frequently conducted on pooled fecal samples, it is more desirable to conduct individual OPGs as field studies have shown significant variation in oocyst counts between samples from the same flock. Proper interpretation of OPGs must consider differences in the number of oocysts/dose among live coccidiosis vaccines. Likewise, it should be expected that the number of oocysts produced is lower and peaks later when using attenuated vaccines containing precocious strains. With the most widely used non-attenuated live coccidiosis vaccines, depending on the Eimeria spp., oocyst counts are low at the beginning (6-8 days), peaking around 15 to 18 days and quickly declining thereafter.
The Hybrid or Bio-Shuttle Program
It seems paradoxical to use an anticoccidial in conjunction with a live coccidiosis vaccine susceptible to all anticoccidials, but the reality is that some companies do just that. This program aims to take the edge off the peak shedding and lesions to improve live performance parameters without interfering with active immunity development. However, this practice could potentially predispose flocks to NE issues. Achieving this is a difficult balancing act since the timing is critical, and anticoccidials administered via the feed are added to a fixed feeding schedule. In an ideal situation, the anticoccidial would not be added before 21 days. Still, many companies switch to grower feed at or soon after 14 days. Therefore, care must be taken to ensure that the anticoccidial inclusion rate is not too strong to interfere with immunity development. In general, synthetic anticoccidials are stronger than ionophores, but they are the only ones that can be used in a hybrid program for RWA chickens, so selecting an anticoccidial medication and inclusion rate (for those with a dose range) becomes very important. Although ideally, no anticoccidial should be used in programs where the chickens have been vaccinated with a live coccidiosis vaccine, the performance benefits from a bio-shuttle program with ionophores have been demonstrated.
Many alternative feed additives range from triterpenoid saponins, essential oils, organic acids, yeast cell walls, and other natural compounds found in plants that have demonstrated to have some anticoccidial activity. The alternatives mentioned above are more likely to support a live coccidiosis vaccine program without interfering with the development of active and uniform immunity.
Summary
In the US, anticoccidials belonging to the ionophore class are considered antibiotics and cannot be used in RWA programs making claims about raising birds without antibiotics. Therefore, producers must learn to effectively prevent coccidiosis without the ionophore anticoccidials that have been the backbone of coccidiosis prevention programs worldwide for many decades. As a growing percentage of broiler production becomes RWA, veterinarians and producers must learn to effectively prevent and control coccidiosis using innovative techniques and alternative medications. For RWA production, synthetic anticoccidials and live coccidiosis vaccines can be used to prevent coccidiosis. For organic production, only live coccidiosis vaccines can be used to prevent coccidiosis. Regardless of the production system, all factors critical to achieving active immunity, from vaccine storage, administration, and management in the field, must be optimized. Producers should utilize all of the tools available by using carefully planned programs and rotations along with improved flock brooding practices. In summary, coccidiosis can be effectively prevented in RWA poultry production, but it requires planning, dedication and attention to management details from the breeder farm and hatchery to the broiler house.