



Approach to Genotype VII Newcastle Disease with a high incidence in the Middle East Region and North Africa
Newcastle disease (ND) is caused by virulent strains of the Newcastle disease virus (NDV), also recently designated as the Avian Orthoavulavirus 1 (AOAV-1).It is one of the most devastating disease of poultry and wild birds. Phylogenetic analyses clearly distinguish historical isolates (obtained prior to 1960) from currently circulating viruses of class II genotypes V, VI, VII, and XII to XVIII.
Genotype VII is predominant in the Middle East Region (Radwan et al. 2013).
Thus, there have been studies to assess the efficacy of live vaccines such as HIPRAVIAR® CLON (HIPRA®, Spain) against Genotype-VII Newcastle virus challenge. The study entitled “Comparative Efficacy of Two Combined Cloned Vaccines Against Newcastle Disease Virus and Infectious Bronchitis, in a Controlled NDV Challenge (Genotype VII) in Laying Hens¨ (Area Newcastle, HIPRA®) included another commercial vaccine, both of them having shown protection against Genotype VII, although, HIPRAVIAR® CLON presented milder post-vaccine reactions, which reflects, obviously, a higher level of safety.
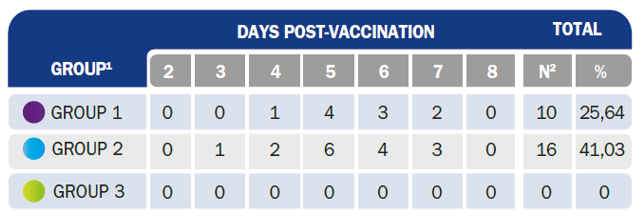
G1 = vaccinated (HIPRAVIAR® CLON/H120), G2 = vaccinated (Nobilis® ND CLON 30/Ma5), G3 = not vaccinated. Total number of birds per group = 39
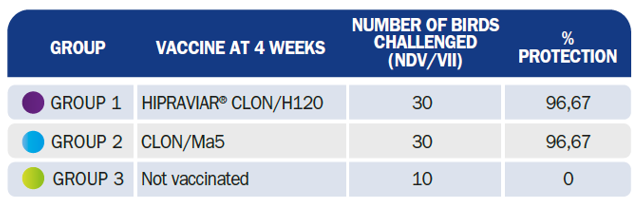
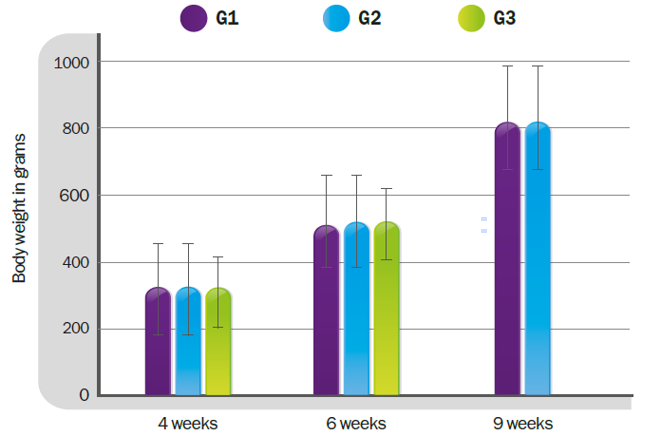
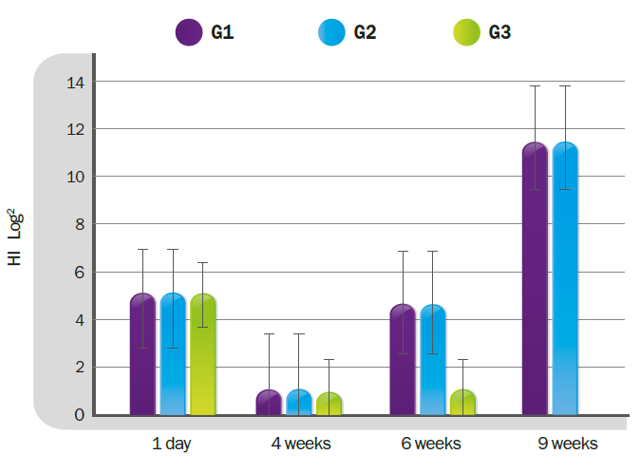
There is a great deal of debate and research concerning Genotype-VII and chimeric vaccines which aim to provide “homologous protection”. These recombinant vaccines included F (glycoprotein responsible for fusion) and HN (hemagglutinin and neuraminidase) genes from genotype VII in a LaSota, a ND virus classed as Genotype II. The basis of such vaccines, however, remains a LaSota virus. This simply means, in other words, that there is no point in a homologous live vaccine, and the reason for this is:
Live vaccines have an impact on local protection, stimulating secretory IgA and cell-immunity mediators (Maraqa, 1996).
The homology makes more sense in the case of inactivated vaccines, because it is more important to have good antibody titres, which reflect humoral protection, and a homologous vaccine would only further reduce the shedding of the virus. A trial performed by Cornax et al., (Avian Diseases, 2012) showed the heterologous protection conferred by LaSota and other commonly used live vaccines.
Furthermore, Genotype VII has several sub genotypes (VIIa, VIIb…), so even the "homology" itself is relative and the concept of "perfect matching protection" is then debatable...
The F and HN glycoproteins are the most antigenic parts in the conformation of AOAV-1, where F is, for example, the criterion for pathotype classification (Wulanjati, 2018). It is critically linked to speed of cleavage in cells. So, if we ever have a Genotype-VII virus, which contains a "LaSota" F protein, this virus will finally replicate at the same speed as a lentogenic virus and in specific host-cells!
Again, in the case of the chimeric vaccine LaSota with Genotype VII insertion, genes for Genotype VII were changed and modified to reduce virulence. But in the end, this does not mean that they are necessarily as immunogenic as the LaSota reference, or better, Cloned LaSota virus.
A trial by HIPRA® involving another Genotype (GXII) and using different live vaccines showed the importance of the live vaccine in the protection of the birds and the effect on production. (Poster WVPA 2019 Thailand - Comparative Protection of Different Vaccination Programmes Against Newcastle Disease in Commercial Laying Chickens Before and After Challenge)
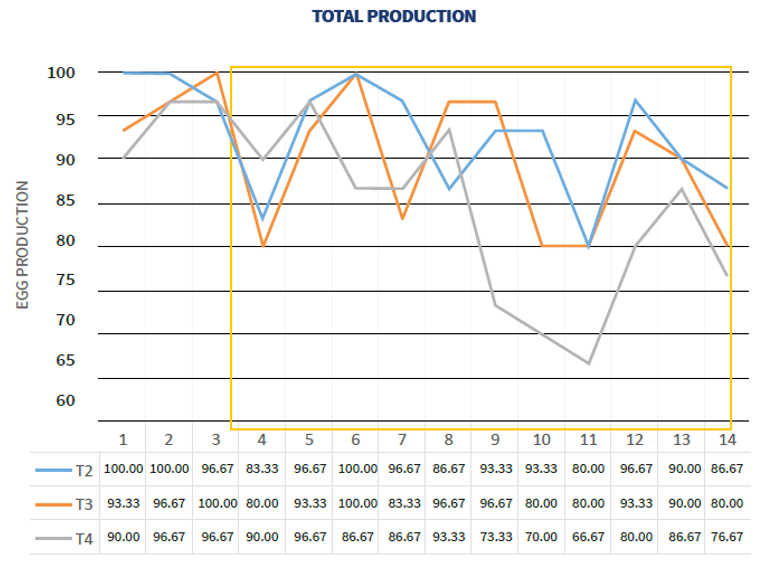
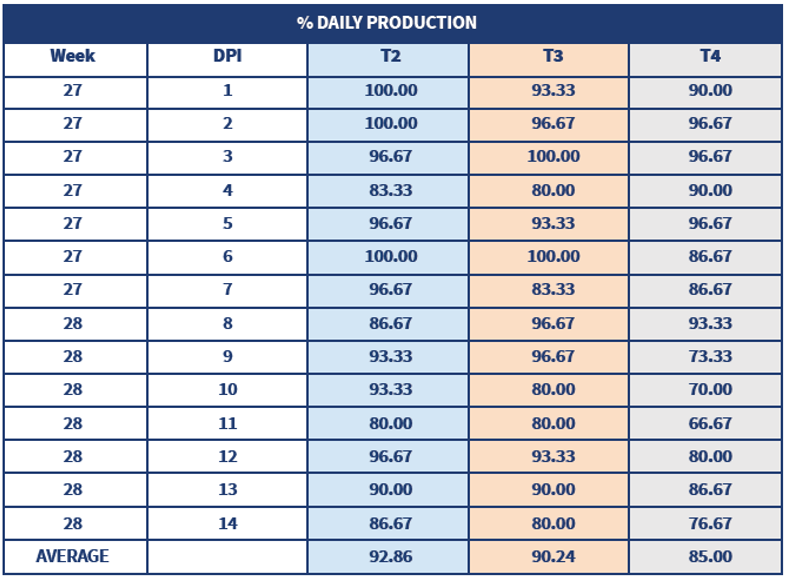
The same trial showed the effect of a live vaccine as a primer to humoral response and that a good live vaccine choice is crucial to protect birds. (Poster WVPA 2019 Thailand - Comparative Efficacy of Different Vaccination Programmes against Newcastle Disease in Commercial Laying Chickens)
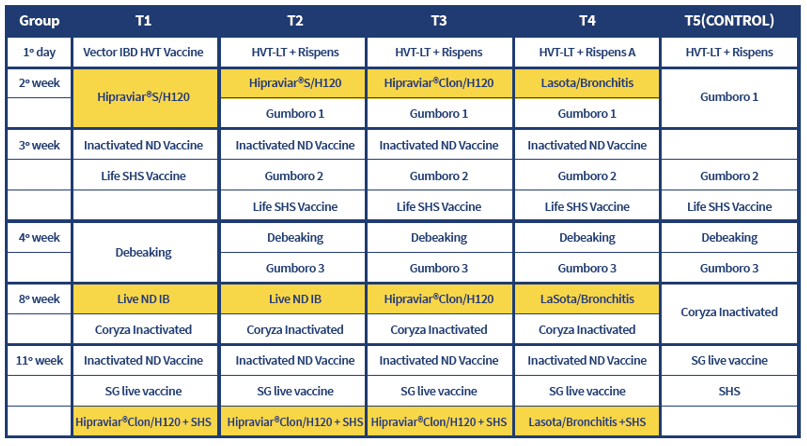
*The inactivated voccinated for Newcastle disease were the same in all four groups.
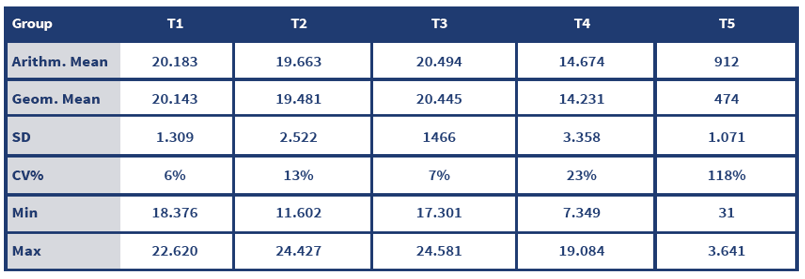
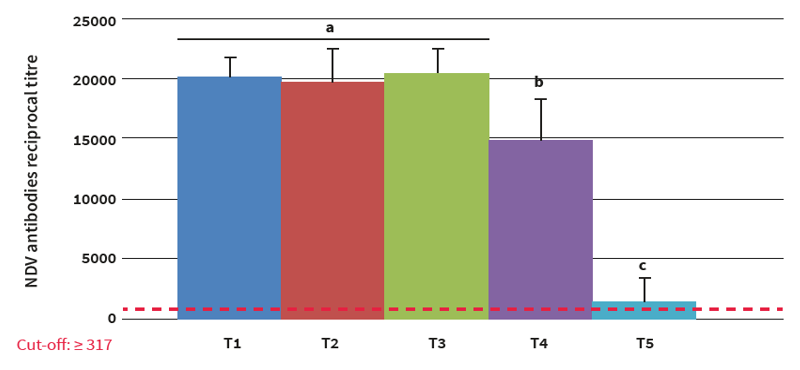
To conclude, all AOAV-1 belong to the same serotype, regardless the genotypes, and this is the reason why we don't really need a homologous vaccine. The antibody titres levels, though, that will make the difference.
| References | ||||
|---|---|---|---|---|
| Cornax I, Patty JM, Afonso CL. | ||||
| (2012) | Characterization of live LaSota vaccine strain–Induced protection in chickens upon early challenge with a Virulent Newcastle Disease Virus of heterologous genotype.. Avian Diseases, | |||
| Diel DG, da Silva LH, Liu H, Wang Z, Miller PJ, Afonso CL. | ||||
| (2012) | Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. | |||
| Dimitrov KM, and al. | ||||
| (2019) | Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. | |||
| Maraqa, AD. | ||||
| (1996) | Studies on the immune response to Newcastle disease virus in poultry.. Iowa State University Capstones, Theses and Dissertations | |||
| Radwan, MM., Darwish, SF., El-Sabagh, IM. et al. | ||||
| (2013) | Isolation and molecular characterization of Newcastle disease virus genotypes II and VIId in Egypt between 2011 and 2012.. Virus Genes | 47, 311–316 | ||
| Wulanjati, MP., Wijayanti N. and Haryanto A., | ||||
| (2018) | Phylogenetic analysis of Newcastle disease virus from Indonesian isolates based on DNA-sequence of fusion protein-encoding gene.. Biotechnology, | 17: 69-74. |









