Assessing the economic burden of avian infectious bronchitis on poultry farms in Brazil
Demonstrating a a significant and measurable economic impact on the broiler and breeder industries.by L.P. Colvero (1)*, L.Y.B. Villarreal (1), C.A. Torres (2) & P.E. Brandão (2)*
(1) MSD Animal Health Brazil, Condomínio Rochaverá Corporate Towers, Avenida das Nações Unidas 14171, CEP 04794-000, São Paulo, Brazil (2) Department of Preventive Veterinary Medicine and Animal Health, School of Veterinary Medicine, University of São Paulo, Av. Prof. Dr Orlando M. Paiva, 87, CEP 05508-270, São Paulo, SP, Brazil *Corresponding authors: [email protected] and [email protected]
Summary
Avian infectious bronchitis (IB), caused by avian infectious bronchitis virus (IBV), is a worldwide endemic disease of chickens that affects all branches of the poultry industry. Multiple geno/serotypes occur, and low vaccine cross-protection results from the highly divergent IBV types.
In view of the lack of consistent data on the economic losses caused by IB and the poor protection resulting from the use of the Massachusetts type as a live vaccine in Brazil, this survey aimed to estimate the losses per 1,000 birds in broiler and breeder flocks positive for IBV.
Thirty-two different IBV genetic types were found. In breeders, the total loss per 1,000 birds was US$3,567.4 and US$4,210.8 at 25–26 and 42 weeks old, respectively, whereas in broilers (48 days old), the estimated loss was US$266.3 per 1,000 birds.
Taken together, the results show a significant and measurable economic impact on the broiler and breeder industries, with an age-dependent increasing trend and an association with multiple genetic types of the virus.
Introduction
Avian coronavirus (avian infectious bronchitis virus [IBV] in chickens), the causative agent of avian infectious bronchitis (IB), is a Gammacoronavirus that spreads by aerosol transmission and replicates in epithelial cells in the respiratory, reproductive and enteric tracts, and in the kidneys (1).
Consequently, IBV infection can have many clinical manifestations, such as delayed growth, decreased egg production, eggshell malformations and infertility, and it may lead to condemnation of a large number of carcasses in slaughterhouses (1).
Infectious bronchitis is an OIE-listed disease that is globally recognised as one of the diseases of most concern because of its economic effects on commercial poultry production and its potential for negative effects on trade (2), such as results from the spread of virus types among countries.
In Brazil, IB outbreaks in vaccinated flocks are very common and are caused by Brazilian field strains of a serotype divergent from vaccine strains of the Massachusetts serotype (3), the only live IBV vaccine type approved by the Brazilian Ministry of Agriculture (MAPA). However, no reliable data are available on the economic losses caused by IBV in Brazil.
Therefore, the aim of this survey was to estimate the economic losses in flocks of broilers (pre-slaughter) and breeders affected by IBV, and to determine the diversity of IBV types found in these birds.
Materials and methods
Sample collection
From 2012 to 2014, 49 flocks of Cobb breeders, layers and broilers (Table I) with a reported occurrence of IB were sampled for IBV screening on poultry farms from different regions of Brazil. The birds were humanely euthanased, and samples of the respiratory tract (lungs and trachea), kidneys, caecal tonsils, enteric contents, testes and female reproductive organs were collected. All birds had been vaccinated with at least one dose of an attenuated Massachusetts IB vaccine and variable booster schedules, including inactivated IBV Massachusetts and D274 strains. No signs compatible with avian influenza or Newcastle disease, which could also affect the production indexes, had been observed in the surveyed flocks.
Avian infectious bronchitis virus typing
The samples were tested using a nested reversetranscription polymerase chain reaction (RT-PCR) targeting the 3' untranslated region (UTR) of IBV (4) after total RNA extraction with TRIzol™ (Life Technologies), using M-MLV™ (Life Technologies) and GoTaq™ (Promega) for the cDNA synthesis and PCR steps, respectively, as per the manufacturers’ instructions.
Positive samples were subjected to a nested PCR targeted to the partial amplification of the S1 subunit of the IBV spike gene (nucleotides 28–477 of the S gene of the M41 strain, GenBank accession number DQ834384.1), as described by Torres et al. (5).
The amplicons (450 bp) were purified using EXOSapIt and subjected to bi-directional sequencing with BigDye 3.1™ (Life Technologies) and an ABI 3500 Automatic DNA Sequencer™ (Applied Technologies). The sequences were checked for Phred scores (only values ≥ 21 were used), assembled with CapContig in Bioedit 7.0.9.0 (6) and aligned with homologous sequences retrieved from GenBank using Clustal/W in Bioedit 7.0.9.0 (6). Finally, an amino-acid neighbour-joining phylogenetic tree, using the Poisson model and 1,000 bootstrap replicates, was built with Mega 6 (7).
Estimation of economic losses
Broilers
Parameters
The parameters used for the estimation of economic losses in broiler flocks in this study were weight and mortality. Standard values for the Cobb 500 line were compared with parameters of the flocks sampled in the field. In this study, the cost considered when a broiler is delivered to a slaughterhouse was US$0.97 per kg. All the expected numbers for breeders and broilers were extracted from the Cobb Handbook.
Economic loss
The number of dead birds during IB outbreaks was separated from the total number of dead birds as follows:
1. Total number of birds dead during IB outbreaks = Total number of dead birds – Number of birds dead due to expected mortalityThe weight loss was estimated in two parts, according to the equations below:
2. Total weight (in kilograms) not delivered to the slaughterhouse due to broiler mortality during the IB outbreak = Total number of broilers dead during IB outbreaks × Expected weight per broiler to be delivered to the slaughterhouse (Cobb standard)
3. Total weight (in kilograms) not delivered to the slaughterhouse due to decreased weight gain of broilers during the IB outbreak = Total weight expected to be delivered to the slaughterhouse – Total real weight of broilers delivered to the slaughterhouse
The economic loss per 1,000 broilers was estimated in US$ according to the equation:
4. Economic loss per 1,000 broilers = (Equation 2 + Equation 3) × 0.97/Number of housed birds/1,000
Breeders
Parameters
The parameters for the estimation of economic loss in broiler breeder flocks in this study were the percentage of production lost, mortality and the number of chicks produced. These parameters for the flocks in the field were compared with standard values for the Cobb 500 line. In this study, the costs considered were US$0.4 per chick, US$0.2 per egg and US$0.97 per kilogram. The standard expected weight of a breeder at 45 weeks was considered to be 3.011 kg.
Economic loss
The first parameter analysed was the mortality due to IB, calculated for breeders according to Equation 1, above.
The cost of IB mortality was based on how many chicks a breeder failed to produce:
5. Cost of breeder mortality due to IB = Total number of birds dead during IB outbreaks per week × Expected number of chicks that should be produced until 65 weeks of age
Another factor that was analysed in this study was the decrease in egg production during the IB outbreak, considered from when an above-expected mortality rate was noticed until two weeks after the mortality rate was again considered normal.
6. Number of eggs (chicks) not produced due to IB = (Expected number of eggs – Observed number of eggs produced) × Standard hatching rate
The economic loss per 1,000 breeders in US$ was estimated according to the equation:
7. Economic loss per 1,000 breeders = (Equation 5 + Equation 6) × (3.011 kg × $0.97)/Number of birds/1,000Owing to the lack of complete and/or reliable data from the field, the economic impact of IB was not estimated for layers.
Results
Avian infectious bronchitis virus typing
Thirty-two different IBV genetic types were found among the 49 flocks, each flock being positive for at least one genetic type (Fig. 1 and Table I); all of them belonged to the Brazilian genotype described by Villarreal et al. (8). The corresponding sequences have been assigned GenBank accession numbers KF679836–KF679840, KF679842– KF679845, KF679849, KF679852–KF679853, KF679855, KF679857, KF679859–KF679861, KF679864–KF679865, KF679872, KF679875, KF679883 and KP719937– KP719946.
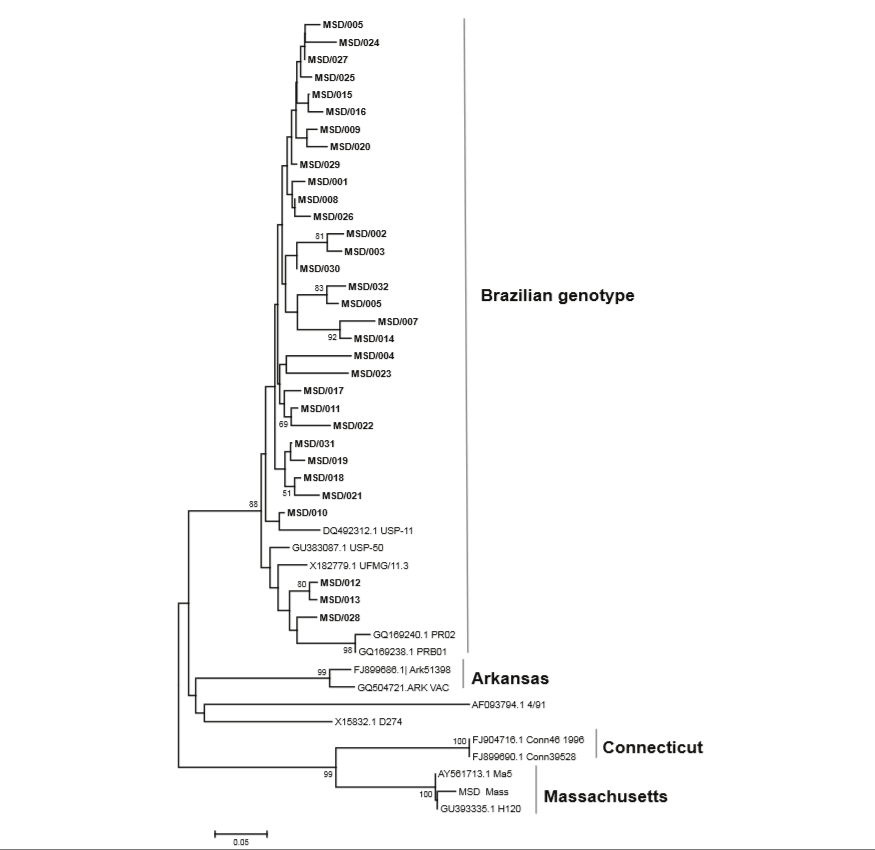
Fig. 1 Amino-acid neighbour-joining maximum composite, likelihood (NJ MCL) tree for the partial sequences of the S1 subunit of the avian infectious bronchitis virus spike gene. Sequences from this study are in bold Numbers at each node are bootstrap values (1,000 replicates; only values > 50 are shown). The bar is the number of substitutions per site
The mean amino acid identity amongst the 32 Brazilian genetic types was 91.8% (standard deviation [sd] 3.3). When compared with the other genotypes shown in Figure 1, these 32 Brazilian genetic types showed the highest mean amino acid identity with D274 (75.6%, sd 1.6) and the lowest with Connecticut (61.7%, sd 1.1). When compared with Massachusetts, the amino acid identity was 68.4%, sd 1.8.
Table 1. Genetic types of avian infectious bronchitis virus (based on partial S1 amino acids) found in this study in different types of bird, with year of detection, total number of occurrences for each genetic type in all samples tested, the frequency of each genetic type and the number of flocks for each type.
.PNG)
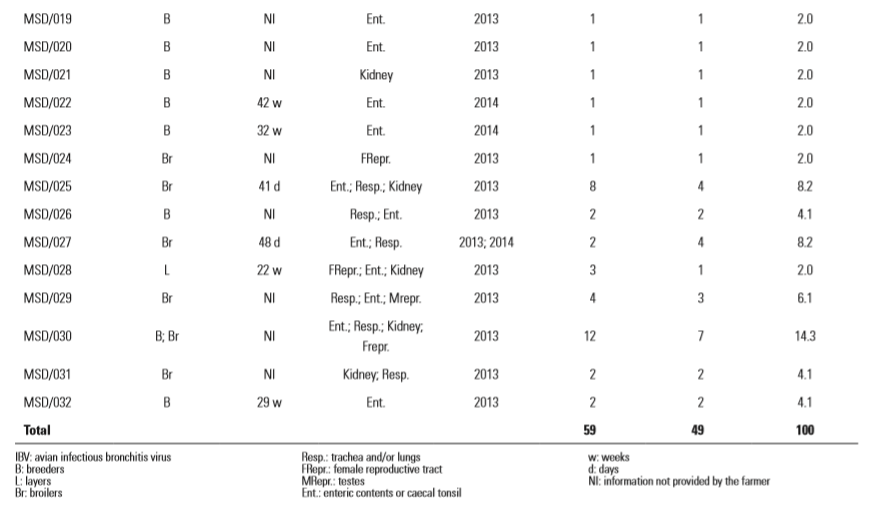
As the S1 segment used in this survey is not homologous to that used for the definition of the BR1, BR2, BR3 and BR4 genetic types, the definition of subtypes was not possible.
The most common of these genetic types was MSD/30, found in 14.3% of the flocks, in breeder and broiler samples of enteric content/caecal tonsils, respiratory tract, kidney and female reproductive tract. Most of the sequences (n = 24) were exclusive to a single flock (Table I).
Estimation of economic losses
In breeders from 25 to 26 weeks of age in which IBV genetic types MSD/001–004 were found, the total loss per 1,000 birds per flock was estimated as US$3,567.4. In breeders from 28 to 32 weeks of age with genetic types MSD/008, MSD/015, MSD/023 and MSD/32 the estimate was US$4,221.5/1,000 birds. For older breeders (42 weeks) in which genetic type MSD/022 was found, the estimated loss per 1,000 birds was US$4,210.8. In 48-day-old broilers, a total loss of US$266.3 was estimated per 1,000 birds in which the genetic type MSD/027 was detected. These results are summarised in Table II.
For the other flocks presented in Table I, the economic loss could not be estimated because of the lack of reliable data from the farms, including one of the two broiler farms shown in Table I.
Discussion
As shown in the present survey, there is a large number of genetic types of IBV in Brazil. This diversity has been previously reported in this country and all the genetic types described herein cluster in a well-established Brazilian geno/ serotype which is spread all over the poultry-producing areas in the country (8, 9).
The core problem regarding the control of IB in Brazil is the fact that currently only live attenuated IB vaccines of the Massachusetts serotype are used. Given that such vaccines have already been shown to confer poor protection against the Brazilian types of IBV (10), the lack of vaccine protection allows the emergence of IBV types that can readily replicate in chickens and spread easily, causing disease in the affected birds.
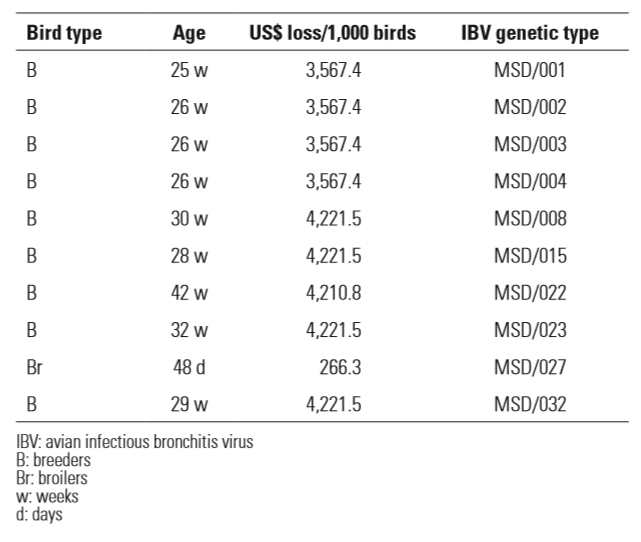
The economic loss per 1,000 birds for all ages and bird types presented in Table II represents a great burden for the farmers because a consistent part of their profit is lost as a result of IB. It is of note that, for breeder flocks, the losses increase by 17% from the beginning (25 weeks) to the end (42 weeks) of the productive life of the birds, meaning that a cumulative effect is found.
In the 48-day-old broilers, at the final stage of life, the losses are lower than those found for breeders (Table II), but this is a biased comparison because i) the reproductive signs of IB are not manifested in broilers and ii) the profit margin in broiler production is lower when compared with that for layers and breeders.
In an earlier study on the economic impact of IB in Brazilian poultry (11), the economic losses in breeders were estimated as US$251.4/1,000 birds and in broilers US$8.46/1,000 birds, values that differ greatly from the ones obtained in the present study. The main reasons for such a difference may be that the authors of the previous report based their estimations only on clinical signs of IB without a definitive diagnostic test, which may have led to a biased classification of flocks as IB-positive. In addition, a single Brazilian region was sampled in their study, whereas in the present study a diverse geographical area was surveyed with multiple field virus types and management procedures.
Despite the multiple occurrences of some of the IBV genetic types described herein and the economic losses in birds in which some of these genetic types were found, it was impossible to correlate differences in losses with given IBV genetic types. This is because the diversity within geno/serotypes is low, but the amount of cross-protection decreases with amino acid distance (12).
Given the genetic distance of the IBV types described herein from the Massachusetts type, which is the only type of live IB vaccine allowed for vaccination in Brazil, in general these economic losses may be attributed to poor vaccine protection.
In conclusion, IBV causes a significant and measurable economic impact in broiler and breeder flocks, with an age-dependent increasing trend and an association with multiple virus genetic types that undergo positive selection when vaccine-induced immunity is poor.
References
1. Jones R.C. (2010). – Viral respiratory diseases (ILT, aMPV infections, IB): are they ever under control? Br. Poult. Sci., 51 (1), 1−11. doi:10.1080/00071660903541378.
2. Bagust T.J. (2013). – Poultry health and disease control in developing countries. Poultry development review. Food and Agriculture Organization of the United Nations, Rome, 96–100.
3. Villarreal L.Y., Brandão P.E., Chacón J.L., Saidenberg A.B., Assayag M.S., Jones R.C. & Ferreira A.J. (2007). – Molecular characterization of infectious bronchitis virus strains isolated from the enteric contents of Brazilian laying hens and broilers. Avian Dis., 51 (4), 974–978. doi:10.1637/7983-041307.1.
4. Cavanagh D., Mawditt K., Welchman D.B., Britton P. &
Gough R.E. (2002). – Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol., 31 (1), 81–93. doi:10.1080/03079450120106651.
5. Torres C.A., Tonietti P.O., Hora A.S., Taniwaki S.A., Villarreal L.Y.B. & Brandão P.E. (2014). – Avian coronavirus in quail: continued surveillance after the introduction of a vaccination program. In Proc. 8th Symposium on avian corona & pneumoviruses and complicating pathogens, 2nd Annual Meeting of the Cost Action FA1207, 17–20 June, Rauischholzhausen, Germany, 77–86.
6. Hall T.A. (1999). – BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp. Ser., 41, 95–98. Available at: brownlab. mbio.ncsu.edu/JWB/papers/1999Hall1.pdf (accessed on
19 November 2015).
7. Tamura K., Stecher G., Peterson D., Filipski A. &
Kumar S. (2013). – MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molec. Biol. Evol., 30 (12), 2725–2729. doi:10.1093/molbev/mst197.
8. Villarreal L.Y., Sandri T.L., Souza S.P., Richtzenhain L.J., de Wit J.J. & Brandao P.E. (2010). – Molecular epidemiology of avian infectious bronchitis in Brazil from 2007 to 2008 in breeders, broilers, and layers. Avian Dis., 54 (2), 894–898. doi:10.1637/9218-121709-Reg.1.
9. Di Fabio J., Rossini L.I., Orbell S.J., Paul G., Huggins M.B., Malo A., Silva B.G. & Cook J.K. (2000). – Characterization of infectious bronchitis viruses isolated from outbreaks of disease in commercial flocks in Brazil. Avian Dis., 44 (3), 582–589. doi:10.2307/1593097.
10. De Wit J.J., Brandao P., Koopman R. & Villarreal L.Y.B. (2014). – Report of the genotyping, pathotyping and protectotyping of IBV strains from Brazil. In Proc. 8th Symposium on avian corona and pneumoviruses and complicating pathogens, 2nd Annual Meeting of the Cost Action FA1207, 17–20 June, Rauischholzhausen, Germany, 300–310.
11. Assayag M.S., Chacón J.L., Rocha P.T. & Kuana S. (2012). – Economic impact of infectious bronchitis in a Brazilian poultry integration system. In Proc. 7th Symposium on avian corona and pneumoviruses and complicating pathogens, 18–21 June, Rauischholzhausen, Germany, 80–83.
12. Jackwood M.W. (2012). – Review of infectious bronchitis virus around the world. Avian Dis., 56 (4), 634–641. doi:10.1637/10227-043012-Review.1.