



Auditing Vaccine Application Procedures in Poultry
By J.H. Breytenbach, Intervet International b.v. - Millions of Euros are invested annually in the vaccination of poultry. A vaccines efficacy however is dependant on effective administration. What tools are available to effectively monitor and audit vaccination techniques? This paper will discuss the requirements for an effective vaccination, focusing on the differences between live and inactivated vaccine preparations. Proposals for the practical auditing of the vaccination procedure are discussed with the goal of improving vaccine administration and ultimately bird immunity.
The Goal
Dr Edward Jenner introduced the world to the concept of vaccination in 1796. With his somewhat crude experiment, performed on his gardener's eight-year-old son, Jenner proved that a Cowpox vaccination induced immunity to the more severe disease Smallpox. Science may have progressed since those early days, but Jenner's observations are still valid.
To induce immunity an antigen (Cowpox) must be presented in a sufficiently high enough dose to initiate an immune response in the target species.
There is a time delay from point of vaccination to the development of protective immunity (Jenner waited 8 weeks before challenging with Smallpox).
Modern poultry farming has resulted in the development of high-density poultry areas, which bring with them an increased risk of disease spread. The industry manages this risk by routine vaccination against known poultry pathogens of specific economic importance. The efficacy of a vaccination schedule however requires proper vaccine administration, a challenging feat when thousands of birds need to be vaccinated at one time.
Vaccines used in the poultry industry are either live attenuated viral/bacterial strains or inactivated viruses/bacteria formulated with a suitable adjuvant. Reputable vaccine manufacturers subject all batches of vaccines to stringent quality control tests, ensuring the end user is supplied with a product containing sufficient antigen to initiate an immune response in the target bird. Thus assuming bird health, vaccine administered to the target bird in the prescribed dosage will induce immunity to the specific disease. Furthermore, a well-planned vaccination schedule will result in maximum immunity at the time when poultry are most susceptible to infection or when disease would have the highest negative impact on economic performance.
Live vaccines
Live poultry vaccines are generally attenuated bacterial or viral strains, which replicate in the vaccinated bird inducing a cellular and humeral immune response. Due to the ability to self propagate most live vaccines are suitable candidates for mass application by spray or via the drinking water. Mass application is however not without potential risks. Poor administration techniques result in only a section of the population being exposed to the vaccine strain with resultant poor flock immunity.
Success of live vaccination is also dependant on the vaccine strain being presented to the correct target cells. This is clearly demonstrated when comparing the efficacy of a live Newcastle Disease (ND) vaccine administered by different routes. Gough and Alexander demonstrated immunity to a ND virus challenge within 3 to 4 days of a ND vaccination administered by the aerosol route, an administration technique that delivers the vaccine virus directly to the respiratory mucosa. In comparison administering the same vaccine by the drinking water route resulted in a delay in the development of immunity (Refer Graph 1). Further to this Gough and Alexander demonstrated that the aerosol route of administration induced the highest serological response. (Refer Graph 2).
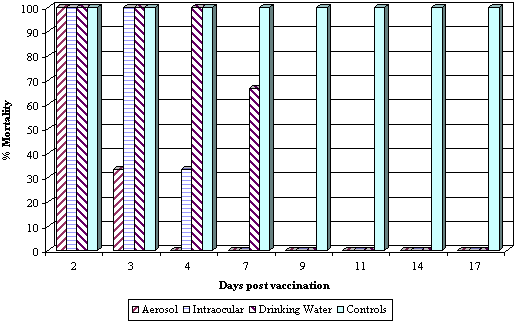 Graph 1: Percentage mortality in chickens challenged by aerosol at various intervals with a virulent ND strain (Essex '70), following day old vaccination with a live ND B1 vaccine administered by different application methods. (Adapted from Gough and Alexander, 1973). |
Inactivated vaccines
Inactivated vaccines are formulated with a high antigenic mass of bacterial or viral origin formulated in a suitable adjuvant. Vaccination results in a humeral response, the magnitude of which is directly related to the concentration of antigen administered per dose (Refer Graph 3). Inactivated vaccines must be administered by injection; success of vaccination is thus dependant on the skills of the vaccine administrator in administering a full dosage of vaccine to each bird. This too holds true for certain live vaccines that are administered by injection.
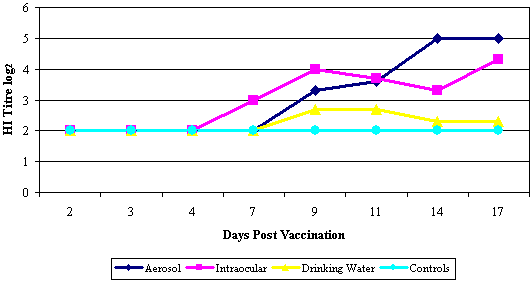 Graph 2: Haemagglutination inhibition titres of sera from chickens vaccinated with a live ND B1 administered by different application methods. (Adapted from Gough and Alexander, 1973) |
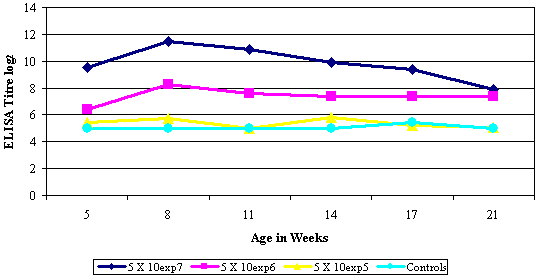 Graph 3: ELISA titres for Ornithobacterium rhinotracheale (Serotype B) measured at various time intervals. Three groups of chickens were vaccinated at two weeks of age, each with a batch of O. rhinotracheale containing a different concentration of bacterial cells. A fourth group remained unvaccinated as negative controls. |
Auditing Vaccination Procedures
Auditing of vaccination procedures should be implemented on two levels. Firstly, on farm auditing of the accuracy and efficacy of the actual vaccine application procedures, and secondly a retrospective evaluation based on the interpretation of serology results.
For the purpose of this paper we will discuss methods to audit each administration route separately, followed by a section on the interpretation of serological results.
Drinking Water Administration
The goal of a drinking water vaccination is for each chicken to drink a minimum of one dose of vaccine solution. On the surface this may appear to be the simplest method of live poultry vaccine mass administration; however a drinking water application done correctly is time consuming.
Audit point one: Water availability
Vaccine should be reconstituted in a sufficient volume of water to cater for a two-hour period.
Sufficient drinker space is required to allow free access to the vaccine solution.
Water consumption during the first three weeks of a chick's life is erratic, thus walking through the house during the vaccination chasing up inactive chicks, especially along the sides of the house, is recommended.
To further stimulate drinking birds should be deprived of water prior to the vaccination. Usually 1 to 2 hours of water deprivation is sufficient to stimulate thirst, however this is dependant on environmental conditions.
The water system must be drained prior to administering the vaccine solution, this to avoid the first chickens quenching their thirst on standard water.
Audit point two: Vaccine viability
The cold chain must be maintained during the transportation of vaccine to the site and on site prior to use.
Avoid exposure of vaccine vials and vaccine solution to direct sunlight.
To prevent neutralisation of the vaccine virus/bacteria drinking water must have a neutral pH and be free of disinfectants (including chlorine), detergents and heavy metals.
The addition of skimmed milk or skimmed milk powder to the water 20 - 30 minutes prior to adding the vaccine virus/bacteria is recommended as a stabilizer.1
Reconstitute vaccine in a smaller quantity of water (5-10 liters) before adding to the drinking water. This ensures a homogenous vaccine solution.
Vaccine solution must be consumed within 2 - 3 hours of reconstitution.
Audit point three: Assessing water intake
To evaluate the uniformity of water intake a blue dye is added to the vaccine solution (Appendix 1). The crops and tongues of birds that have consumed vaccine solution will be temporarily stained blue. The intensity of the colouring is an indication of the volume of water consumed. A correct vaccination procedure results in at least 90% of chickens colouring blue.
1 Recommend 2.0 grams of skimmed milk powder per litre of water or 2.0 litres of skimmed milk per 100 litres of water.
Spray Administration
The spray application technique is especially suitable for the administration of respiratory type vaccines such as Newcastle Disease and Infectious Bronchitis, as the vaccine strain is deposited directly onto the target cells; respiratory mucosa. We distinguish between two forms of spray with different goals and applications.
Coarse Spray is intended to deliver large droplets of vaccine to the upper respiratory tract and eye. In addition the feathers of the bird are moistened stimulating preening which further increases the chance of vaccine uptake. Coarse spray is the preferred method of vaccine administration for young unprimed chicks.
Fine Spray or mist is intended to hang in the environment of the birds and is inhaled into the respiratory tract. The smaller droplet size allows for a deeper penetration into the lower respiratory tract of the bird. This method of vaccine administration is suitable for older birds that have previously been primed to a specific respiratory antigen.
Audit point one: Vaccine viability
The cold chain must be maintained during the transportation of vaccine to the site and on site prior to use.
Avoid exposure of vaccine vials and vaccine solution to direct sunlight.
As smaller volumes of water are used with spray applications (200 - 1 000 ml per 1 000 doses) it is recommended to preferably use distilled or de-ionised water.
Where distilled or de-ionised water is not available care must be taken that water used has a neutral pH and is free of disinfectants (including chlorine), detergents and heavy metals. The addition of skimmed milk or skimmed milk powder to the water 20 - 30 minutes prior to adding the vaccine virus is recommended as a stabilizer.
Audit point two: Equipment
The correct equipment must be selected for the desired application technique. In practical terms a spray is considered coarse when droplets generated fall to the ground as would be seen with a shower of rain. A fine spray hangs in the air forming a mist above the birds.
Equipment must be free of disinfectants and detergents, and should preferably be dedicated for the purpose of vaccine application.
Cleanliness of equipment is paramount especially where equipment is being shared among different sites.
Audit point three: Vaccine distribution in the poultry house
Uniform vaccination requires a uniform distribution of the vaccine spray in the poultry house at bird eye level.
Vaccine must be reconstituted in a sufficient volume of water to distribute the required number of dosages over the entire population.
Birds in open barn style housing must be crowded for an effective vaccination. Crowding is achieved using barriers or by herding birds to the sides of the house forming a bird free passage down the center.
Birds in cages are sprayed cage by cage.
During vaccination ventilation must be turned off and open sided houses must have curtains raised to keep out cross winds. This is especially important when fine spray or mist is the method of application. In excessively hot climates minimum ventilation may be required, in which case application should be limited to a coarse spray.
Spray distribution and droplet size can be monitored using water sensitive paper, a special paper which changes colour when exposed to moisture. Place strips of water sensitive paper in the back of cages or against the wall to test that vaccine spray is being distributed to all corners of the house/cage.
Administration by Injection
All inactivated as well as a few live poultry vaccines are administered by a subcutaneous or intramuscular injection. This method of application requires accurate deposition of the full dosage of vaccine to each individual bird. Technically this is the most demanding vaccination technique as operators are required to work at pace while vaccinating thousands of birds. The immune response to inactivated vaccines is dose related, thus a large percentage of birds receiving a partial dose or being missed will result in a poor immune response for the flock (Refer Graph 3). The importance of inactivated vaccine application cannot be over emphasised as inactivated vaccines administered to breeder type birds often have a direct impact on the performance of their offspring.
Audit point one: Equipment
Suitable reliable equipment. Various multi-dose syringes are available on the market; the brand selected must be compatible with mineral oils often used as adjuvants.
Syringes must be calibrated prior to use and at regular intervals during vaccination procedure. Use of an accurately calibrated test tube is recommended for this purpose.
Syringes and all ancillary equipment must be sterilized prior to use.
The correct size needle must be selected according to age of birds being vaccinated, site of injection and type of vaccine being administered.
Needles should be regularly replaced, at least once every thousand birds.
Blunt needles and needles with burs must be replaced immediately.
Audit point two: Vaccination technique
Physically check vaccinated birds for:
deposition of vaccine at the correct site,
wet feathers indicating that vaccine was poorly administered; either full dose or partial dose in feathers due to premature or delayed expulsion of vaccine from the syringe.
A blue dye may be used when administering vaccines reconstituted in a phosphate buffered saline type diluent, such as Mareks Disease vaccines. This improves the visibility of the vaccine once administered. Use of a blue dye in mineral oil based vaccines is however not recommended as the blue dye may persist for an extended length of time resulting in condemnations at slaughter.
Audit point three: Balance the books
Reconcile the doses of vaccine used to the number of birds vaccinated. Overdosing is costly, under-dosing results in poor immunity.
Serology is a helpful tool in evaluating the efficacy of an individual operator. Checking the serological response to a specific antigen administered by the operator will indicate the accuracy of vaccination. A poor %CV in combination with a low mean titre could indicate a large percentage of birds are being missed or are not receiving the full vaccine dose (Refer section on serology).
The key to vaccine administration by injection is: "Quality is more important than speed!"
Serology
Serology provides a useful profile on previous stimulation of the bird's immune system, but serology by no means provides the full picture. Serology only measures circulating antibody levels (IgG and IgM), a function of humeral immunity, and fails to give us insight into cellular and local immunity. Despite this shortcoming, used in the correct context serology can provide valuable clues on the success or failure of a vaccination.
The key to reliable serology results start on the farm with sample collection. For sampling to be statistically valid there are two basic conditions that have to be met:
Random selection of birds for sampling
This implies that every bird in the flock should have an equal chance of being selected for sampling. Collecting all samples from one corner of a house could give a false result if vaccine distribution was not uniform.
A protocol should be agreed upon whereby samples are collected throughout the house.
Proper sample size
The number of blood samples taken from a flock has a direct impact on the reliability of the results. The fewer the number of samples collected the higher the risk of calculating an inaccurate mean flock titre.
Twenty-three samples is the minimum recommended number to be collected for a meaningful appraisal of flock immunity.
In addition to the above factors sample handling and storage is important to ensure a good quality serum sample is delivered to the laboratory. Samples with excessive haemolysis, bacterial or fungal contaminants or samples that have started to decompose will not deliver reliable serology results (Refer to appendix 2 for guidelines on proper sample handling)
Interpretation of Serology following Vaccination
Following vaccination it generally takes 4 - 6 weeks for the development of significant antibody levels. Earlier sampling is possible, but peak antibody levels will not yet have been achieved especially when evaluating the serological response to inactivated vaccines. To interpret seroconversion two parameters must be evaluated.
The mean titre of the tested birds in a flock tells you how strong the antibody response is. A low mean titre could indicate samples were collected to soon after vaccination, or a poor vaccine application, or in the case of inactivated vaccines poor priming prior to vaccination.
The coefficient of variation percentage (%CV) provides an indication on how variable the titre response of a flock is. For most diseases the %CV following a correctly applied vaccination should be less than 40%. If the %CV is above 60% there is definite room for improvement of the vaccine application techniques.
Summary
The success of a vaccination is dependant on correct vaccine administration.
Vaccine administration is best audited on site at point of application.
Checking on correct procedures
Using tools such as blue dye or water sensitive paper to evaluate uniformity of administration
Retrospectively the success of a vaccine administration can be evaluated by interpreting serology results.
Attention to the details of administration is required to achieve a consistent immune response following vaccination.
References
Cserep, T. "Intervet Poultry Division Datafile - Administration of Inactivated Vaccines" (2002). Intervet UK Limited.
Cserep, T. "Intervet Poultry Division Datafile - Drinking Water Vaccination" (2002). Intervet UK Limited.
Cserep, T. "Intervet Poultry Division Datafile - Spray Vaccination of Poultry" (2002). Intervet UK Limited.
Löhren, U. "Diagnosis of vaccination failures in broilers" (2002). International Poultry Production. Volume 10 Number1.
Gough R.E. and Alexander D.J. "The Speed of Resistance to Challenge Induced in Chickens Vaccinated by Different Routes with a B1 Strain of Live NDV" (1973). The Veterinary Record, May 26th, 1973.
Source: Intervet UK Ltd - May 2005









