



Betaine And AGP- free Diets
By Milan Hruby and Janet Remus, published by eFeedLink - Betaine, through its ability to preserve intestinal integrity, will help reduce intestinal lesions in conjunction with a coccidiostat, and thus may aid in minimising losses due to sub-clinical and clinical necrotic enteritis.Published work has also shown an association between E. maxima infection and increased caecal Clostridium perfringens number (Waldenstedt et al., 1998). In work where E. tenella has been the dominant parasite there seems to be little proliferation of Clostridium perfringens (in fact the opposite) in the caeca (Figure 1).
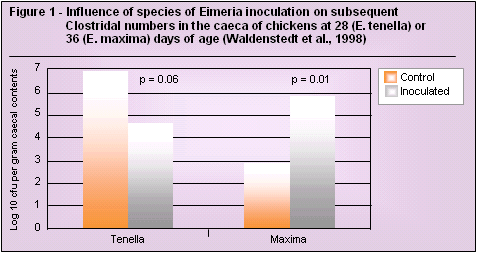
The reason for such a specific relationship may lie in the fact that E. acervulina, E. maxima and E. necatrix inhabit the site of major digestion and/or absorption i.e. the small intestine, whereas E. tenella inhabits the caeca.
It is proposed that the proliferation of Clostridium perfringens and development of necrotic enteritis is facilitated by the appearance of large quantities of dietary protein in the caeca. Certainly feeding poorly digested nitrogen has been shown to favour production of butyrate (an indicator of Clostridial fermentation) in the caeca of rats (Morita et al., 1998). Experimental models for inducing necrotic enteritis either involve feeding very high levels of protein or duodenal infusion of Clostridia which have been grown on high protein media (horse flesh). This suggests that clostridia need to be exposed to high levels of nitrogen in order to become virulent. Under normal circumstances, nitrogen is well digested, little enters the caeca and necrotic enteritis is uncommon.
However, when viscous diets are fed or coccidiosis increases small intestinal damage, excessive quantities of nitrogen escape digestion and enters the caeca resulting in a more frequent occurrence of necrotic enteritis (AI Sheikhly and AI Saieg, 1980; Kaldhusdal and Skjerve, 1996; Riddell and Kong. 1992). Sloughed cells from damaged villi will provide nitrogen to the caeca in addition to the undigested feed. It is thus evident that E. tenella may not so readily precipitate necrotic enteritis since, although it will provide damaged caecal enterocyte nitrogen to the caeca, it does not affect digestion in the small intestine. Reducing the damage that coccidiosis causes to the small intestine is essential if nitrogen digestibility is to be maintained and necrotic enteritis minimised.
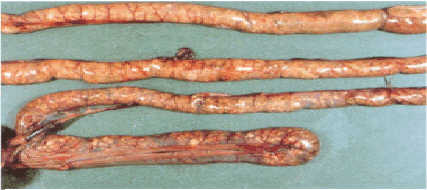
Anterior small intestine. Several affected mucosa with light
lesions visible through the serosal surface.
Betaine improves the osmotic condition of the enterocyte (Allen et al. 1998), and when fed with a coccidiostat it appears to reduce the incidence and severity of coccidial lesions thus improving animal performance (Figure 2). In our study (Finnfeeds, 1996) it is clear that betaine per se had no direct effect on lesion score in isolation. In the presence of a coccidiostat, however, it is apparent that improving the osmotic status of the gut epithelium through addition of betaine results in further reductions in lesion scores. In other reported studies, betaine plus salinomycin significantly inhibit invasion of both E. tenella and E. acervulina.
However, subsequent development of E. acervulina is inhibited more effectively than that of E. tenella. (Augustine et al., 1997). Recent work (Waldenstedt et al., 1999) showed no interaction between narasin and betaine in birds which had been inoculated principally with E. tenella and E. acervulina, which seems in contrast to the benefits seen in figure 2. However, the same study did not show any benefit of narasin alone, suggesting the species used were resistant to the coccidiostat. This reinforces the supposition that betaine, through its function as an osmolyte, is effective in potentiating a coccidiostat, but ineffective as a replacement.
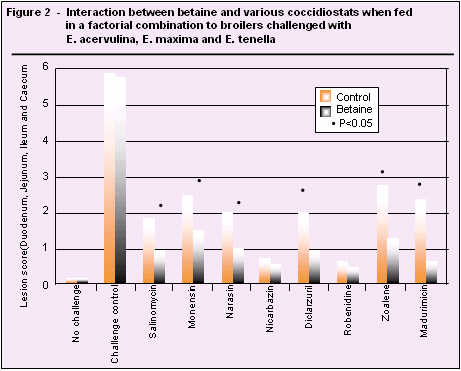
Thus, if the coccidiostat in itself is effective, the response to added betaine is likely to be positive. If the coccidiostat is ineffective, as in the above study, then betaine is unlikely to provide additional benefit.
Betaine, through its function as an osmolyte can enhance the ability of a coccidiostat to minimise intestinal damage caused by small intestinal species of Eimeria. As a result, digestive integrity of the intestinal tract is better maintained and less nitrogen can enter the caeca. Limiting substrate for Clostridium perfringens growth is essential if necrotic enteritis is to be kept to a minimum, since the absence of antibiotic growth promoters will allow for a rapid establishment of the disease if favourable conditions exist.
Removal of antibiotic growth promoters is likely to increase variability in broiler performance. When unrestrained intestinal microfloral populations are combined with undigested feed substrates, their numbers will increase substantially. The poorer the quality (digestibility) of the feed and the greater the microbial challenge (environment) the greater this problem will be.
References
Allen. P.C., Danforth, H.D., and Augustine, P.C. (1998) lnt.J. Parasitol.: 28 (7) p. 1131-1140
AI Sheikhly, F. & AI Saieg, A. (1980) Avian Dis. 24:324-333.
Augustine, P. C., Mcnaughton,J. L, Virtanen, E., & Rosi, L. (1997) Poult. 7:1623-1623.
Elwinger, K., Berndtson, E., Engstrom, B., Fossum, 0., & Waldenstedt, L (1998) Acta Vet 39:433-441.
Hock, E., Halle, I., Matthes, S., & Jeroch, H. (1997b) Agribiological Research-Zeitschrift fur Agrarbiologie Agrikulturchemie Okologie 50:85-95.
Kaldhusdal M. & Skjerve, E. (1996) Preventive Veterinary Medicine 28: 1-16.
Morita. T., Kasaoka. S., Oh-hashi, A., Ikai, M., Numasaki, Y., & Kiriyama. S. (1998b) J.Nutr. 128: 1156-1164.15&1 164. Riddell, C. & Kong, X.-M. (1992) Avian Dis. 36.,499-503.
Waldenstedt, L., Elwinger, K. Hooshmand-Rad, P., Thebo, P., & Uggla, A (1998) Acta Vet. 39:461-471. Waldenstedt, L., Elwinger. K., Thebo. P., & Uggla, A. (1999) Poult. Sci. 78:182-189.
Source: eFeedLink - February 2005









