Coccidiosis and welfare-friendly production systems for laying hens: A new connection
The last decade has without doubt been the most revolutionary ever regarding animal welfare in the poultry industry, especially in some parts of the world. It has been so in all the poultry production sectors, both meat and eggs. However, the egg sector has probably undergone the biggest changesBy Martina Dardi, DVM, MSc, Brand Manager, Poultry BU HIPRA. Spain
In Europe, - where consumer trends are more demanding - countries have clearly chosen cage-free production. For example, in The Netherlands, Germany, Austria and Sweden everything or almost everything is produced in cage-free systems in response to clear consumer demands. In fact, the poultry system has become one of the fundamental criteria when it comes to choosing what to buy at the supermarket.
Definitively, this global welfare demand comes more strongly from the food companies/retailers than the legislators themselves and this is clearly a consequence of consumer pressure for cage-free eggs. In 2015, McDonalds announced that all its eggs in the U.S and Canada would come from cage-free hens. This announcement was followed by many similar ones from other food companies and retailers.
The evolution of these production systems with an ever-increasing percentage of laying hens that are floor-reared or reared in systems like aviaries also has an influence on diseases and how we will have to prevent them. Specifically, preventing coccidiosis in these systems has already become one of the many challenges that egg producers will have to face in the near future. In laying hens it is fundamental to maintain the best intestinal health and, as a consequence, preventing coccidiosis will be one of the main focuses.

Example of free range cage-free laying hens. Source: Hipra
The greatest evidence of this new situation is that coccidiosis vaccination in laying hens has increased considerably in the EU but also outside the EU. Many egg producers have taken the decision to vaccinate all their flocks against coccidiosis, as the disease has started to appear more frequently in these semi-floor type rearing systems.
But why is that and what has changed compared to before? Well, the answer is simple, but comes with challenges at the same time.
Simple, as coccidiosis appears everywhere that birds have the opportunity of coming into contact with their faeces and pecking them. Ten or fifteen years ago, when most of the laying hens were reared in conventional cages, coccidiosis hardly occurred at all. It was very rare that the birds had the chance to come into contact with their faeces and a few cases appeared on those occasions when hens could peck the manure belt of the floor above.
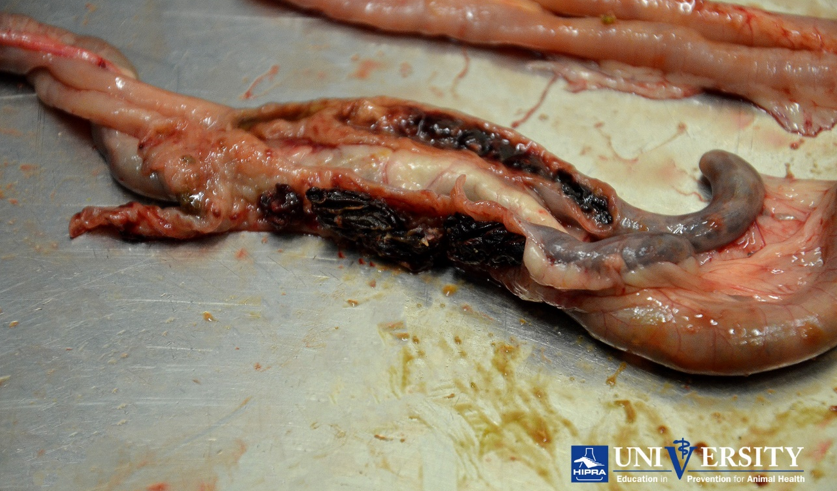
Eimeria tenella lesion. Source: HIPRA.
So how do laying hens come into contact with their faeces nowadays? It is clearly the case with hens reared on the floor or in aviary systems, where hens are reared totally or partially on the floor. However, it could also be the case with hens reared in furnished (enriched) cages as some faeces can accumulate in some areas of the cage, such as the nests, and don’t fall on the manure belt below the cage. Here again, when the hen pecks the faeces, it has the opportunity to come into contact with Eimeria oocysts as they are ubiquitous. Every contact with faeces exposes the hen to a possible coccidiosis challenge.
Eimeria necatrix lesion. Source: HIPRA.
This is why nowadays we need to work on coccidiosis prevention in all the three above- mentioned production systems: floor-reared, aviary systems and sometimes also with enriched cages. It also has to be said, that the use of anticoccidials for coccidiosis prevention in laying hens is sporadic as they need to be used constantly for almost the whole rearing period and this does not prove to be cost-effective in the end. Conversely, vaccination is well recognised as the most convenient prevention strategy in laying hens, as one vaccination can protect birds for their entire lives, as long as certain conditions are met. And this is where the challenges lie:
- Choice of vaccine
- Day of vaccination & route of administration
- Management after Vaccination
1. Choice of vaccine
Talking about the type of vaccine to use, one must take into account 2 essential points:
Attenuation degree. Most of the Eimeria vaccines available nowadays consist of live parasites that need to undergo two and sometimes three entire life cycles inside the host gut in order to trigger the immune system and subsequently establish a full protective immunity. On the market, there are live non-attenuated and attenuated vaccines. Live non-attenuated vaccines consist of parasites that still maintain their natural virulence. Control of the development of adverse reactions (coccidiosis disease) is achieved through the use of low numbers of oocysts in vaccine preparations and in some cases even by the use of anticoccidials to control the excessive spread of vaccine strains. Live attenuated vaccines are specifically designed to generate an immune response whilst limiting the threat of possible adverse events and putting the birds in a better position to achieve the objectives of uniformity and weight during the rearing period. A lack of attenuation and low degree of attenuation may lead to deterioration of the gut health of vaccinated animals.
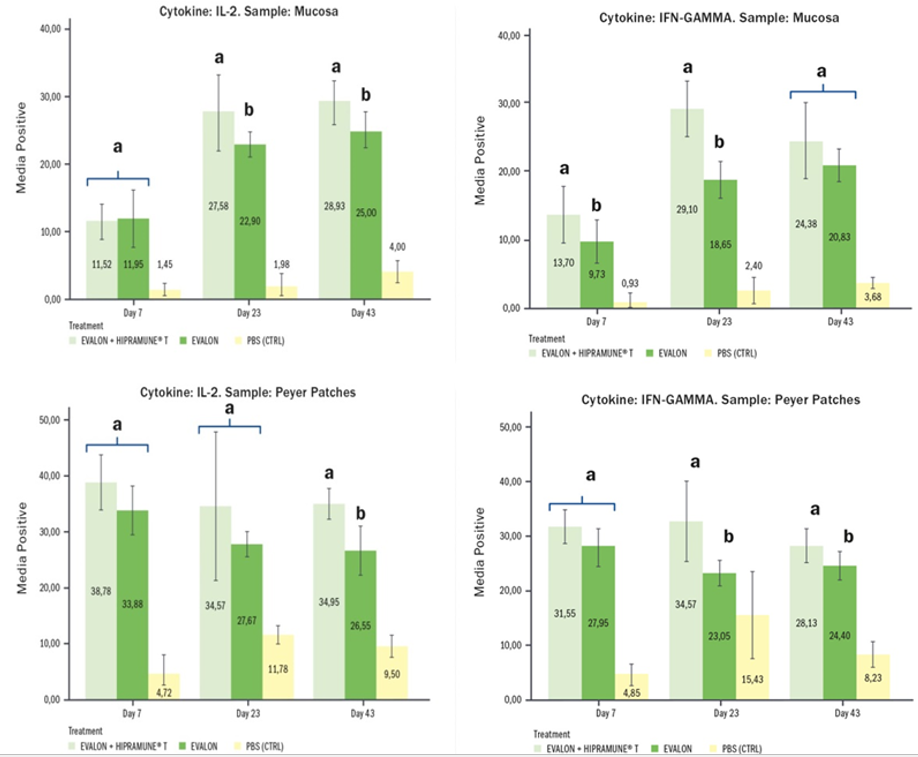
Immunomodulation. This is a new concept introduced recently in coccidiosis live vaccines. But why the use of an immunomodulator? On the one hand, we know that coccidiosis vaccines are able to elicit a robust protective immunity after ingestion and re-cycling of the vaccine oocysts in the litter. However, very often this re-cycling can be hindered – as already seen - by several factors: dry conditions of the litter, low densities or production facilities (e.g. aviary systems, slats, etc...). It is exactly under these circumstances that the presence of an immunomodulator is of paramount importance as it can drive the development of the immunity towards a cell-mediated one even when the correct replication of the vaccine cannot take place completely. On the other hand, in order to survive, the Eimeria parasite fights back. In fact, it has evasion mechanisms that are able to play with immunity and, in this way, it is able to elicit a type of immunity that is not protective and effective (Jang et al. 2011, Schmid et al. 2014, Miska et al. 2013). The objective of the adjuvant is to make the vaccine work better, as it triggers the type of immunity – cell-mediated - that is important for a protective immune response against Eimeria. Specifically, Pagès et al.2 (2015) saw that the action of the adjuvant used in EVALON®’s HIPRAMUNE®T led the immune response towards more effective immune mechanisms: the Th1 and Th2 responses need to be balanced and this is what the immunomodulator does. Results obtained from experimental trials indicate that HIPRAMUNE®T is able to increase the level of Th1 cytokines, as indicated by the results obtained for IL-2. Regarding IFN-gamma, statistically significantly higher levels were also detected on different days both in the mucosa and Peyer’s patches. In contrast, levels of IL-4 and IL-10 were equal or lower when the group receiving EVALON® plus HIPRAMUNE®T was compared to the group receiving EVALON® alone. These results confirm the ability of HIPRAMUNE®T to stimulate a cellular immune response.
Mean percentage of positive results for IL-2 and IFN-gamma. Results obtained with lymphocytes isolated from the mucosa and intestinal Peyer’s Patches. Superscripts (a,b) indicate statistical differences (Pagès et al.2 2015).
2. Day of vaccination & route of administration
Generally speaking, coccidiosis vaccines can be administered at any age, however the sooner the better, as we want the birds to be fully immunised when the field challenge starts to occur, which is usually around 4-5 weeks of age. For this reason, but also for practical reasons, it is best to vaccinate at 1 day of age. In fact, especially in those cases where the hens are partly or wholly reared in cages – as in aviary systems or enriched cages - it is very difficult to perform the vaccination when hens are already housed inside the cages.
There are several methods by which coccidia vaccines can be administered, but the first thing we need to keep in mind is that coccidiosis vaccines have to be ingested orally in order to start the 1st replication in the gut:
- Spraying the vaccine directly on to the feed risks desiccating the oocysts and that is one of the few weak points of oocysts. Also, it is not practical when birds are reared in cages as the worker will have to open each cage individually;
- Drinking water is suitable for this type of vaccine, but it has to be with bell drinkers. This practice, apart from being time-consuming and tedious, is very rare nowadays as bell drinkers have almost disappeared, giving way to nipple drinkers. Nipple drinkers tend to be very long – especially in layer houses - and this makes oocysts deposit at the bottom and they can also be retained along the nipple mechanism. Together with the fact that inside nipple lines it is very common to find biofilm that can also trap the oocysts and prevent them reaching the tip of the nipple for ingestion;
- The vaccine can also be applied by spray on the farm at the time the chicks are received and are still inside the boxes. This method is also effective but it is time-consuming for the staff and there is inconsistency between farms, as there are several models and types of portable sprayers or backpacks. It can be counterproductive for the day old chicks because water and feed withdrawal are increased after an already long journey;
Spray on boxes at the arrival on farm. Source: courtesy of Aranzazu Trueba Palau
- Spray over cages (for enriched cages and aviary systems): this is probably the least advisable method as immediately the hens hear the spray noise, they start to move and as a consequence most of the vaccine is lost over the equipment inside the cage, thus we don’t know really how much vaccine the hens will ingest;
- Probably the most convenient, consistent, reliable and accurate way - for all the three laying hen production systems mentioned so far - is via coarse spray in the hatchery with a dedicated device. In fact, this method makes it possible to keep the critical points under control in order to ensure a proper vaccine intake and the subsequent development of immunity:
- Dilution of the vaccine to ensure a correct dose/chick: we need to be sure that each chick ingests the correct amount of oocysts and for this to occur, the vaccine has to be diluted in the right amount of water, not too much and not too little. The preparation of the vaccine solution in a dedicated and clean room definitely helps to avoid theses mistakes.
- Uniformity of distribution of the vaccine over the chicks’ trays, which is strictly linked to the volume (ml) sprayed per box: for 100 chick boxes, at least 28 ml is required to ensure that the area of the box is covered.
- Dimension of the droplets (> 200 µm): droplets have to be large enough in order to be detected by the chicks and draw their attention to stimulate pecking. They also have to contain enough water to keep the oocysts hydrated up to the moment of ingestion. To obtain coarse spray droplets we need an adequate nozzle and a device that is able to work at constant pressure throughout the vaccination process. Only in this way we can guarantee the correct dimension of the droplets for each sprayed box. This can only be obtained at the hatchery and working with high precision electric pistons.
- Presence of a magnetic stirrer: it is well known that Eimeria oocysts are heavy in weight and that this can differ depending on the species of Eimeria. This means that the oocysts tend to deposit at the bottom of the vaccination tank as this is a vaccine suspension not a solution. This is why it is important to keep the vaccine suspension thoroughly mixed throughout the vaccine administration and also with different mixing speeds depending on the volume of vaccine suspension contained in the tank.
- Presence of a Colouring Agent and Aroma: uniform ingestion of coccidiosis vaccines is of paramount importance for the correct intake of these vaccines and subsequent onset of immunity and has to take place soon after the vaccine is coarse sprayed over the chicks. In order to achieve this, we need to enhance the pecking and preening behaviour of the chicks with the help of a colour and an aroma. It has been reported that chicks have colour peak preferences in the orange-red and blue-violet regions (Fischer et al., 1975, 1981). In fact, by the addition of a light purple coloration to the vaccine solution, under normal light conditions (200 lux), the birds easily detected the vaccine droplets and thus homogeneity of vaccination was improved (Pagès et al.1, 2015). Unfortunately, the conditions of light intensity in most hatcheries is very poor in the area were chicks are spray vaccinated or in the area where the cages are piled up after vaccination and stored before transportation to the farm. Because of this, the colouring agent was also selected as it was the one that gave better results at a light intensity of less than 100 lux. Moreover, another component was introduced to the colouring agent to solve this problem: an aroma. It is interesting to mention that Burne et al. (1996) demonstrated that birds can respond to odorants and that they first use the visual stimulus and secondly they can use an odour stimulus. Because of this, Pagès et al. 1 (2015) investigated the possibility of introducing an aroma into the final colouring agent in order to make it effective at poor light intensities as well. They finally decided to include vanillin in the formula due to the fact that it increased the number of pecking events in conditions of poor light intensity (80 lux).
Hipraspray® is a spraying device especially designed by HIPRA for the administration of coccidiosis vaccines
3. Management after vaccination
After coccidiosis vaccination, in order for immunity to build, it is essential that two to three replication cycles of the vaccine take place in the host gut, in order to achieve a good level of immunisation.
With live coccidiosis vaccines, we look for cell-mediated immunity, as Eimeria is an intracellular parasite. Specifically, during the first replication only an innate immune response takes place with a general inflammatory response and mucus production; together with the exposure of the Ag by the Antigen Presenting Cells (APC) (macrophages and dendritic cells). These APC expose the Ag to the non-differentiated lymphocytes which will then differentiate into memory cells. During this first phase, only the NK play a role in the identification and inactivation of the enterocytes invaded by the parasite. It is only during the second and third replications – the so called adaptive immune response - that specific T memory Cells - the T cytotoxic cells - target the invaded cells and destroy them whilst still continuing to recruit new memory cells.
After seeing this, it is easy to understand that if the first replication is strictly dependent on vaccination technique, the second and third replications depend on the conditions for oocyst sporulation on the farm. It is well known that Eimeria oocysts are infective only when they are sporulated and that sporulation depends on environmental factors such as temperature, moisture and oxygen. The optimal temperature range is between 25 and 28°C and relative humidity must be around 70%.
Going back to the three production systems for layers mentioned so far, the best conditions for vaccine replication and oocyst sporulation can be achieved only in floor-reared layers, whereas with enriched cages and aviary systems we possibly have the worst conditions either for vaccine replication or for oocyst sporulation. On one hand we need to cover the cage floors with paper – to prevent faeces dropping down on to the manure belt and to allow birds to come into contact with their faeces for the 2nd and 3rd replications - and on the other hand we need to use thick paper (around 220-250 grams) to be able to keep the minimum levels of humidity in the faeces for oocyst sporulation. Also the need for thick paper comes from the fact that it should last for at least 21 days after vaccination to ensure that the vaccine completes at least two replications.
Use of thick paper (around 220-250 grams) for a minimum of 21 days after vaccination to allow the vaccine to complete at least two replications. Source: courtesy of Aranzazu Trueba Palau
Afterwards, in the case of aviary systems when cages are opened and the hens have access to the floor, paper is usually put on the floor to allow further re-pecking of vaccine oocysts and subsequent boost of the immunity.
It is quite clear that in the layer sector “The times they are a-changing”, as Bob Dylan said, and changes in production systems also have an influence on an “old” disease like coccidiosis and in the way we will have to prevent it.
References:
Burne T.H.J., Rogers L.J., 1996. Responses to odorants by the domestic chick. Physiology and Behavior, Vol. 60, No. 6, 1441-1447.
Fisher G.F., Morris G.L., Rusham J.P., 1975. Color picking preferences in white leghorn chicks. Journal of comparative and Physiological Psychology, Vol. 88, No. 1, 402-406.
Fischer G.F., Davis J.S., 1981. Brightness effects on color pecking preferences in dark-hatched domestic chicks. Developmental Psychobiology, 14(3):237-249.
Jang S.I., Lillehoj H.S., Lee S.H., Kim D.K., Pagès M., Hong Y.H., Min W., Lillehoj E.P. (2011). Distinct immunoregulatory properties of macrophage migration inhibitory factors encoded by Eimeria parasites and their chicken host. Vaccine Nov 8; 29 (48): 8998-9004.
Miska K.B., Kim S., Fetterer R.H., Dalloul R.A., Jenkins M.C. (2013). Macrophage migration inhibitory factor (MIF) of the protozoan parasite Eimeria influences the components of the immune system of its host, the chicken. Parasitol Res. May; 112(5): 1935-44.
1Pagès M., Dardi M., 2015. Study of the preening habits of one-day old broiler chicks to establish the components of the colouring agent for the spray application of the vaccine HIPRACOX®. Proceedings of the XIX World Veterinary Poultry Association Congress. Cape Town, South Africa, 158.
2Pagès M., Bech G., March R., Sitjà M., Lillehoj H., Dardi M., Rubio Perez J., del Cacho E. (2015). Live coccidiosis vaccine for breeders and layers (EVALON®) immune modulation and enhancement of immunity by the use of an adjuvanted solvent (HIPRAMUNE®T). Proceedings of the LII Symposium Científico de Avicultura AECA-WPSA. Malaga, Spain, 199.
Schmid M., Heitlinger E., Spork S., Mollenkopf H.-J., Lucius R. and Gupta N. (2014). Eimeria falciformis infection of the mouse caecum identifies opposing roles of IFN-gamma-regulated host pathways for the parasite development. Mucosal Immunology Dec 7; 969–982.