



Construction and Evaluation of an HVT Vector Expressing Laryngotracheitis Virus Genes
M. Esaki ¹ ², L. Jensen ², S. Saitoh ¹, S. Saeki ¹, and K. Moore Dorsey ² ¹ Zeon Corporation, Tokyo, Japan ² CEVA BIOMUNE, Lenexa, KansasSUMMARY
A recombinant turkey herpesvirus with the glycoprotein B (gB) gene of laryngotracheitis virus (Vectormune HVT LT) was constructed. Expression of the laryngotracheitis virus (LTV) gB protein was confirmed by Western blots and a black plaque assay (in situ enzyme-based immunoassay on plaques) using gB-specific antiserum. Vectormune HVT LT was genetically stable after five successive passages in chickens (backpassages) or after passages in chicken embryo fibroblast cells (CEF). Growth characteristics of Vectormune HVT LT in CEF were similar to the turkey herpesvirus (HVT) parent FC126 strain. Vectormune HVT LT inoculated in ovo to specific pathogen free (SPF) embryos at 18 days of incubation or subcutaneously to day-of-age SPF chicks did not cause any clinical signs or any grossly observable lesions associated with Marek’s disease or laryngotracheitis through 18 weeks of age. Also, chickens inoculated with Vectormune HVT LT isolated after five backpassages did not show any clinical signs or grossly observable lesions, demonstrating Vectormune HVT LT does not revert to virulence after backpassages. These results showed that the recombinant HVT expressing the LTV gB protein is genetically stable and safe for use in chickens.
INTRODUCTION
Laryngotracheitis is an acute respiratory disease of chickens characterized by signs of respiratory depression, gasping, swollen heads, and expectoration of bloody exudate1. Laryngotracheitis is responsible for egg production losses and mortality. The etiologic agent of laryngotracheitis (LT) in chickens is laryngotracheitis virus (LTV) or Gallid herpesvirus 1. Laryngotracheitis virus is a member of the family Herpesviridae in the subfamily Alphaherpesvirinae. Like most herpesviruses, LTV may become latent in the trigeminal ganglia and be reactivated from latency. The latency of LTV may be a factor in the reappearance of this disease in intensive poultry production areas of the U.S. The glycoproteins of LTV are important immunogens that can elicit both humoral and cell-mediated immunity. Of LTV glycoproteins, gB has been used in recombinant fowlpox viruses and shown to provide protective immunity in chickens vaccinated with the recombinant fowlpox viruses2, 3.
Turkey herpesvirus or Meleagrid herpesvirus 1 is classified in the family Herpesviridae in the subfamily Alphaherpesvirinae. Non-oncogenic HVT is considered part of Marek’s disease virus (MDV) group and designated as serotype 3 MDV. HVT has been used extensively as a safe and efficacious vaccine against Marek’s disease. Due to the large DNA genome, herpesviruses have been evaluated for use as a viral vector carrying foreign gene(s). For poultry, HVT has been evaluated as a vector for various poultry viral diseases.
In an effort to produce an LT vaccine that protects chicks early in life, we have developed a recombinant vaccine in which the gB gene from LTV was inserted into the HVT genome.
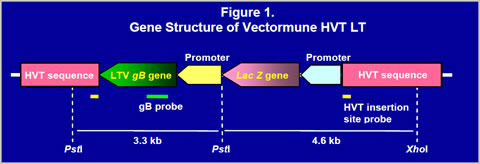
RESULTS
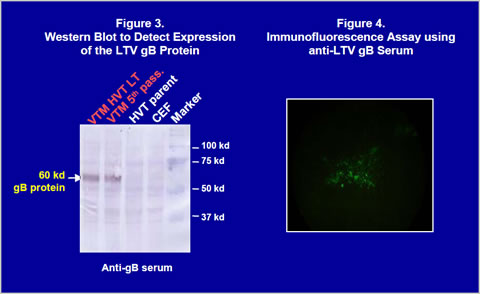
Construction of recombinant virusThe gB gene was isolated from LTV U.S. field strain. The gB gene (1300bp) was obtained by PCR and cloned into a homology vector with HVT insertion site sequences and a promoter (Figure 1). The homology vector also contained the lacZ gene. A recombinant HVT was generated by co-transfecting genomic DNA of the HVT FC126 strain and the homology vector into CEF and purified by β- galactosidase activity. The recombinant virus obtained was confirmed to have an expected gene structure by Southern blot analysis (Figure 2) and sequencing of important regions (data not shown). Vectormune HVT LT expressed a 60 kilodalton protein that can be recognized by goat anti-LTV gB protein antisera in a Western blot (Figure 3). Expression of the gB protein was also confirmed by immunofluorescence assay using gB-specific antisera (Figure 4) and in situ enzyme-based immunoassay on fixed CEF with plaques, called the black plaque assay, using gB-specific antiserum (not shown). Virus after five in vitro passages in CEF was shown to have the same gene structure and express a 60 kilodalton protein from the cloned gB gene (Figures 2 and 3), demonstrating the stability of the virus. The stability was confirmed up to 20 in vitro passages (not shown).
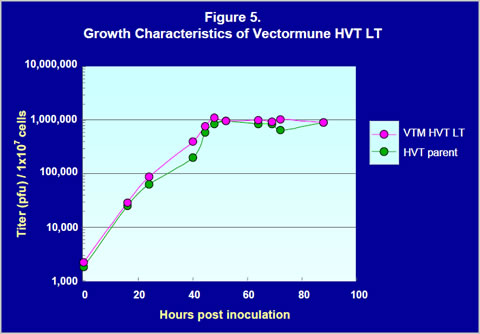
Growth characteristics of Vectormune HVT LT
Growth characteristics of Vectormune HVT LT in CEF were compared with the HVT parent FC126 strain. CEF monolayers in 100-mm tissue culture plates were inoculated with approximately 2000 plaque forming units (pfu) per plate of either Vectormune HVT LT or HVT parent. At ten different time points between 16 and 88 hours after inoculation, cells in the plates were harvested with trypsin and titered. The results of the titration are shown in Figure 5. Titers of Vectormune HVT LT at each time point were equal to or somewhat higher than HVT parent possibly because the inoculum titer of Vectormune HVT LT (2200 pfu/inoculum) was slightly higher than HVT parent (1800 pfu/inoculum). These results indicate that the growth of Vectormune HVT LT in CEF was comparable to HVT parent.
Safety of Vectormune HVT LT
Vectormune HVT LT at 10 x typical dose was inoculated either by in ovo into SPF embryos at 18 days of incubation or subcutaneously into day-of-age SPF chicks. Chickens were observed closely until 18 weeks of age for any clinical signs or adverse reactions. Chickens were then necropsied and observed for grossly observable lesions. No chickens had any clinical signs, adverse reactions, or gross lesions. In the next experiment, Vectormune HVT LT was passaged five times in SPF chickens. Briefly, day-ofage chicks in the first passage were inoculated subcutaneously with Vectormune HVT LT. After one week, blood was taken from the inoculated chickens and used to inoculate another group of day-of-age chicks. This process was repeated five times. At each passage, presence of the virus was confirmed by inoculating CEF with peripheral blood mononuclear cells isolated from the whole blood by Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). Chickens inoculated with the virus at the fifth passage were observed closely for any clinical signs or adverse reactions. Chickens were then necropsied and observed for grossly observable lesions. No chickens had any clinical signs, adverse reactions, or gross lesions. Therefore, it was concluded that Vectormune HVT LT did not revert to virulence after backpassages in chickens. The virus isolated after five passages was propagated and tested by Southern blot, Western blot, sequencing of important regions, and black plaque assay (data not shown). The results obtained from the backpassage virus were identical to the results obtained from the virus before passages. Therefore, the stability of the virus after in vivo passages in chickens was also demonstrated.
Conclusion
Our results showed that recombinant HVT with the gB gene of LTV has the expected gene structure and expresses gB protein. Vectormune HVT LT was stable after in vitro and in vivo passages and replicated in a similar manner as the HVT parent FC126 strain in CEF. Also, the safety of Vectormune HVT LT in chickens was demonstrated
REFERENCES
- Guy, J. S. and T. J. Bagust. Laryngotracheitis. In: Diseases of Poultry, 11th ed. Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald and D. E. Swayne, eds. Iowa State Press, Ames, IA. pp. 121-134. 2003.
- Tong, G. Z., Zhang, S. J., Meng, S. S., Wang, L., Qiu, H. J., Wang, Y. F., Yu, L. and M. Wang. Protection of chickens from infectious laryngotracheitis with a recombinant fowlpox virus expressing glycoprotein B of infectious laryngotracheitis virus. Avian Pathology. 30: 143-148. 2001.
- Sun, H. L., Wang, Y. F., Tong, G. Z., Zhang, P. J., Miao, D. Y., Zhi, H. D., Wang, M. and M. Wang. Protection of Chickens from Newcastle Disease and Infectious Laryngotracheitis with a Recombinant Fowlpox Virus Co- Expressing the F, HN Genes of Newcastle Disease Virus and gB Gene of Infectious Laryngotracheitis Virus. Avian Diseases. 52: 111-117. 2008.









