New approach to old problems – Infectious bronchitis
Infectious bronchitis (IB) is an evolving disease that is present globally and is caused by an avian coronavirus. It is highly contagious and can affect the upper respiratory tract, the reproductive tract, the kidneys, and other organs. It is divided into different serotypes, which can have variable cross protection between them. There are many serotypes, including Massachusetts, QX, Q1, 793/b, Arkansas, BR. The prevalence of the serotypes depends on the geography; for example, the 793/b is prevalent in Europe, and the Q1 in some countries in South America.
A trial conducted by Boehringer Ingelheim [6], demonstrates the advantage of including a variant vaccine, the CR88 strain (a 793/b-like strain), in the vaccination program.
Day-old commercial broilers were obtained from a commercial hatchery. On day 1 they were vaccinated by spray with either H120 strain (BIORAL® H120, Boehringer Ingelheim) or CR88 strain (GALLLIVAC® IB88, Boehringer Ingelheim) following one of two vaccination programs (Table 1). On day 14, the vaccinated groups were re-vaccinated according to the treatment protocol. At 30 days of age, blood was collected for testing by ELISA for IBv antibodies. Selected groups were challenged with one of two virulent Middle Eastern IBv isolates, Is/885 or Is/1494/06 (Figure 1) [2], via the oculonasal route. In the days following challenge, the birds were assessed for clinical signs. At the end of the experiment, the birds were humanely euthanised, and gross lesions in the kidney and trachea were evaluated. Tracheas were collected to measure ciliary protection. Kidney and trachea samples were sampled for histopathology.
Results/Discussion
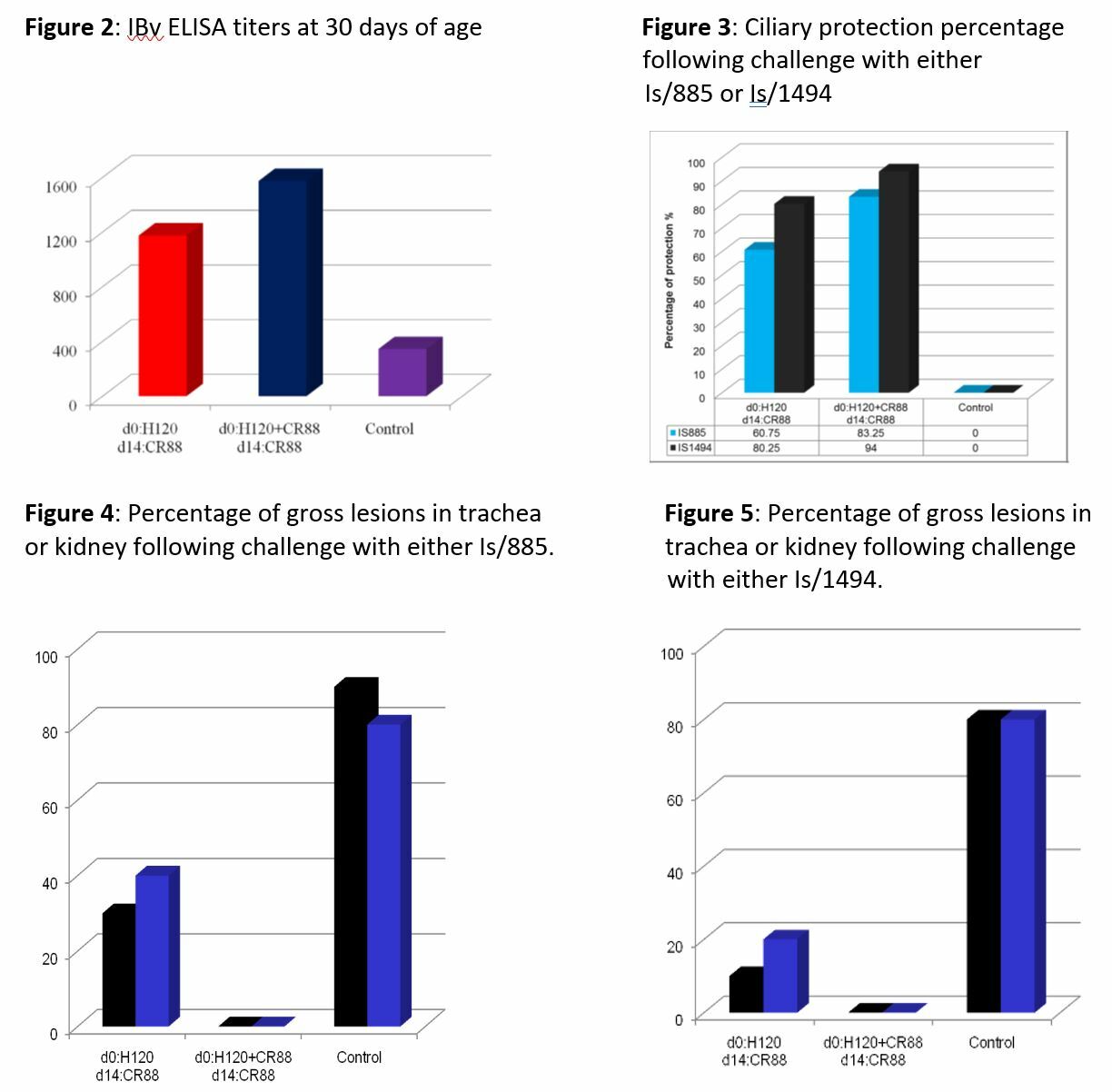
Clinical signs of IB were not observed in either of the vaccinated groups. Observable respiratory signs were recorded in the non-vaccinated, challenged groups at 24 or 48 hours post-challenge, depending on the virus (Table 2). ELISA results showed IBv antibody response levels by 30 days of age in vaccinated groups, while the controls remained low (Figure 2). Ciliary protection measurements remained above 90% for vaccinated-unchallenged and unvaccinated-unchallenged groups (Figure 3). Ciliary protection was improved in terms of response for the Is/1494 isolate, as compared to the Is/885 challenge. Program 2 maintained higher ciliary protection against both challenges. Gross lesions were undetected in birds vaccinated with Program 2 and challenged, as compared to the detection of gross lesions in 20-40% of birds with Program 1 and an Is/885 challenge (Figures 4 & 5).
The two isolates used in this study were clearly virulent strains, showing clinical signs in unvaccinated control birds within 24-48 hours post-challenge. Both vaccination programs afforded protection from clinical signs. A higher percentage of ciliary protection, and absence of gross lesions was provided by the program with H120 and CR88 at one day old, and CR88 again at day 14.
Conclusion
When new IBv variants emerge and become predominant, laboratory work must be conducted to determine pathogenicity and efficacy of available vaccines [1]. In this experiment, two variant Middle Eastern IBV vaccines were assessed against two vaccine programs. A clear advantage occurred in the protection afforded by the program that contained H120 and CR88 at one day old and CR88 again at 14 days. This combination offered improved ciliary protection and lack of gross lesions following challenge.
The above trial demonstrates the importance of the combination of a 793/b-like strain (CR88) with a Massachusetts strain to achieve a broader protection.
THE IB CONTROL
- Diagnostics are of key importance in gaining better understanding the prevalent serotype in the region.
- The use of the vaccines 793/b (Gallivac® IB88 NeO) and Massachusetts strains (Bioral® H120 NeO or Hatchpak® H120) offer cross protection against most of the present IB variants [4].
- The use of IB vaccines can increase the post-vaccine reaction. As a result, it is important to use safer vaccines like the Gallivac® IB88 NeO and Bioral® H120 or Hatchpak® H120.
The Gallivac® IB88 NeO is a 793/b-like vaccine with the strain CR88 121. It is a safe vaccine with a shelf life of 24 months, in which may be used on the same day as Bioral® H120 NeO or Hatchpak® H120 (Massachusetts strain), Gallivac® IBD S706 NeO (intermediate live IBD) and Nemovac (Avian Metapneumovirus) vaccines.
The NeO range is the effervescent tablet vaccine range. It has been developed to improve vaccination practices, making the vaccines easier to store and prepare, safer to use, eco-friendly, and with no compromise on efficacy. Keep in the fridge (between 2° C y 8° C). The pack contains 10 blisters.

The frozen format has greater administration for hatcheries (15,000 doses) and a longer shelf live (36 months). Keep in liquid nitrogen (-196° C).

References
[1] Jackwood M.W., 2012, Review of infectious bronchitis virus around the world, Avian Dis., 56, 634–641.
[2] Awad F., Forrester A., Jones, R.C., Hussein Ahmed H.A., Capua I., Lemiere S., Baylis M., Chhabra R & Ganapathy K., 2013, Immunopathogenesis of infectious bronchitis virus related to IS/1494/06 and IS/885 in specific-pathogen-free chicks, World Veterinary Poultry Association Congress, Nantes, France, 19th-23rd August, pSJC07.
[3] Gelb J. Jr., Wolff J.B. & Moran C.A., 1991. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens, Avian Dis., 35, 82-87.
[4] Ganapathy K., Lemiere S. & Jones R.C., 2009, Immune responses in chicks after single or dual vaccination with live infectious bronchitis Massachusetts and variant vaccines. 16th World Veterinarian Poultry Association Congress, Marrakesh, Morocco, November 8th-12th, p127
[5] Cooper P.W.C. The relationship of Infectious bronchitis virus strain CR88121 to relevant United Kingdom strains. Avian Bull 2_4Aug13
[6] Ganapathy K.1 & Lemiere S.2, 2013, Protection to challenge with infectious bronchitis viruses (IBv) from the Middle East using heterologous commercial vaccine. Avian Bull 3_22Nov13
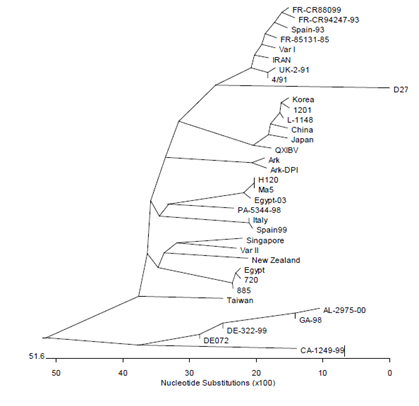
Figure 1: Kimron Veterinary Institute (KVI) Israel – Dr Rosa Meir – IBv phylogenetic tree based on S1 gene sequences of different isolates; isolate Is/885 is close to Middle East (here Egypt) ones; isolate Is/1494 was characterized as an Israel variant II virus; Israel variant I and II viruses are found all over Middle-East countries.
GALLIVAC® and Bioral® are a registered trademark of Boehringer Ingelheim in the United States of America and elsewhere.