



Polyphenols do not represent an equivalent replacement of Vit E
In the opinion of the authors of this article, at the present time it is not justified to postulate overall that supplements containing polyphenols represent an equivalent replacement ofVit E. Vitamin E (Vit E) is the major lipophilic antioxidant in the acid organism and serves above all to protect polyunsaturated fatty acids in cell membranes against oxidative damage. If high doses of Vit E (a-tocopheryl acetate) in excess of needs are supplied, this can influence the lipid peroxidation and certain associated quality parameters of meat (pig, poultry). With regard to possible alternatives to Vit E supplementing, interest is focusing increasingly on the use of flavonoid-rich plant extracts (for example tea extracts) or industrial by-products (for example grape marc) in feed for productive livestock
Authors
Prof. Dr. Siegfried Wolffram, Miriam Luhring
Dr. Ralf Blank, Institute of Animal Nutrition and Physiology
Christian-Albrechts-University of Kiel
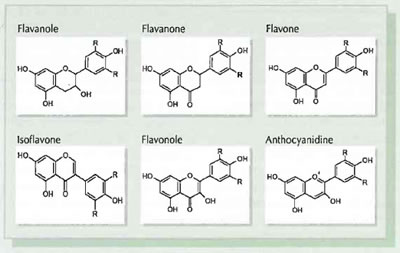
Figure 1: Structural features of the flavonoid subclasses.
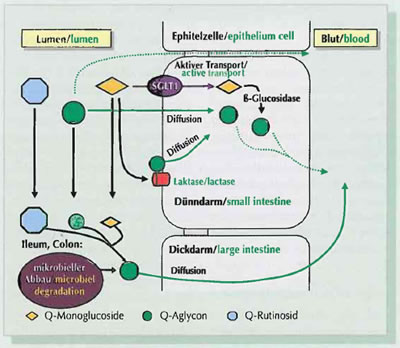
Figure 2: Mechanisms of the intestinal absorption of the flavonol quercetin
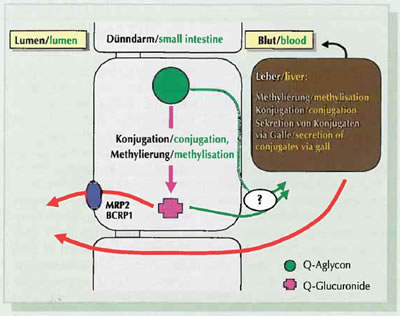
Figure 3: Metabolisation of flavonoid·aglyca (sugar-free) in the small intestine mucous membrane and the liver.
Prof. Dr. Siegfried Wolffram, Miriam Luhring
Dr. Ralf Blank, Institute of Animal Nutrition and Physiology
Christian-Albrechts-University of Kiel
Flavonoids as secondary plant substances are contained in nearly all higher plants. The basic structure oftheflavonoids (Fig. 1) consists of two aromatic and one oxygenated heterocyclic ring. Usually the flavonoids are divided into the following main sub-classes: flavones, flavonols, flavanones, flavan-3-ols (catechins), anthocyanidins and isoflavones. Furthermore, catechins in polymerised form can also be present as proanthocyanidins. Compounds within one subclass differ regarding the functional groups of the ring system.
Bioavailability of flavonoids
An assessment of possible impacts offlavonoids in vivo requires a more detailed look at their bioavailability. Various studies have shown that among other factors the chemical structure, the glycosylation pattern and the molecular weight playa crucial role. As a resultoftheir structural properties by comparison with other flavonoids, anthocyanidins show distinctly poorer bioavailability. Furthermore, distinctly higher bioavailabilityofquercetin monoglucosides was demonstrated in many in vivo studies by comparison with aglycone (sugar-free component) or the quercetin-3- O-glucorhamnoside (rutin). In comparative studies on rats, quercetin showed systemic bioavailability of only nine per cent, while the levels of luteolin, genistein and catechin were 28 to 34 per cent. By contrast with this, data surveyed on rats show a bioavailability of 48 per cent after absorption of 10 mg quercetin-aglycone/ kg live weight (LW). In the case of pigs, a bioavailability of 17 per cent was determined after oral application of 50 mg/kg LW by comparison with intravenous application. There are only relatively few studies on the bioavailability and pharmacokinetics of the proanthocyanidins by comparison with the monomer flavonoids. Studies conducted on sheep and hens showed that radioactively marked proanthocyanidin poly-mers are practically not absorbed and are largely eliminated with the faeces. By contrast, following application of a grape seed extract or chocolate, low concentrations of proanthocyanidin dimers (procyanidin Bl and B2) were demonstrated in human plasma. Absorption of these dimers was 100 times lower by comparison with the absorption of the monomer epicatechin.
To summarise it can be said that depending on the chemical structure, the dose applied, the form of application and the species studied, the bioavailability of flavonoids can lie between 10 and 50 percent.
To summarise it can be said that depending on the chemical structure, the dose applied, the form of application and the species studied, the bioavailability of flavonoids can lie between 10 and 50 percent.
Intestinal absorption
Experiments with rats show that with some flavonoid aglyca (quercetin, genistein, daidzein) slight absorption takes place already in the stomach. Anthocyanins (glycosylised form of the anthocyanidins) form an exception here. They are possibly absorbed intact from the stomach. However, it must be pointed out clearly here that the share of free flavonoids (aglyca) in natural products is generally very low. Yet, aglyca may be partially released during processing of products through heat treatment and other methods. The majority of the flavonoid glycosides reach the small intestine in intact form. A number of studies postulate that the intestinal absorption of the flavonoid glucosides (sugar residue = glucose) takes place in the small intestine, and that of the rhamnosides (sugar residue = rhamnoses or disaccharides and trisaccharides containing rhamnose) in the large intestine.
Owing to the polarity of intact glycosides, absorption through passive, trans-cellular diffusion does not play any role. Instead, it is postulated that various enzymes and transporters are involved here. For instance a number of studies describe that quercetinmonoglucosides (for example quercetin-3-0-glucoside) can be absorbed via the Na+ dependent glucose transporters SGLTl into the small intestine epithelium and subsequently be split in intra-cellularfashion by an unspecific, cytosolic rJ.-glucosidase (Fig. 2). Furthermore, participation of the monocarboxylate transporter during absorption of catechins from the small intestine is discussed. In addition to this the lactase/phlorizin hydrolase (LPH), a I!.-galactosidase located in the brush border membrane of the small intestine epithelium appears to be involved in the absorption of flavonoid glucosides in the small intestine. According to this the LPH catalyses the extracellular hydrolysis of the sugar residue in the absorptive surface of the small intestine epithelium. The aglycone released in this way is then absorbed by passi- ' ve diffusion in the small intestine epithelium. Flavonoid aglyca and flavonoid glycosides (for example rhamnosides) that cannot be absorbed in the small intestine can be absorbed partially from the large intestine (Fig. 2). Microbial enzymes playa crucial role for the deglycosylation here. In addition to the release of aglyca, comprehensive microbial degradation of proanthocyanidin, flavanols, flavonols and isoflavones takes place. The ABC transporters such as for example the "multidrug resistance associated protein 2" (MRP2) and the "breast cancerrelated protein 1" (BCRP1) play an important role. These transport mechanisms reduce the bioavailability of flavonoids, as one of their tasks is to facilitate exporting into the gut lumen of flavonoid conjugates formed in the small intestine mucosa and absorbed into the small intestine mucosa (Fig. 3).
Owing to the polarity of intact glycosides, absorption through passive, trans-cellular diffusion does not play any role. Instead, it is postulated that various enzymes and transporters are involved here. For instance a number of studies describe that quercetinmonoglucosides (for example quercetin-3-0-glucoside) can be absorbed via the Na+ dependent glucose transporters SGLTl into the small intestine epithelium and subsequently be split in intra-cellularfashion by an unspecific, cytosolic rJ.-glucosidase (Fig. 2). Furthermore, participation of the monocarboxylate transporter during absorption of catechins from the small intestine is discussed. In addition to this the lactase/phlorizin hydrolase (LPH), a I!.-galactosidase located in the brush border membrane of the small intestine epithelium appears to be involved in the absorption of flavonoid glucosides in the small intestine. According to this the LPH catalyses the extracellular hydrolysis of the sugar residue in the absorptive surface of the small intestine epithelium. The aglycone released in this way is then absorbed by passi- ' ve diffusion in the small intestine epithelium. Flavonoid aglyca and flavonoid glycosides (for example rhamnosides) that cannot be absorbed in the small intestine can be absorbed partially from the large intestine (Fig. 2). Microbial enzymes playa crucial role for the deglycosylation here. In addition to the release of aglyca, comprehensive microbial degradation of proanthocyanidin, flavanols, flavonols and isoflavones takes place. The ABC transporters such as for example the "multidrug resistance associated protein 2" (MRP2) and the "breast cancerrelated protein 1" (BCRP1) play an important role. These transport mechanisms reduce the bioavailability of flavonoids, as one of their tasks is to facilitate exporting into the gut lumen of flavonoid conjugates formed in the small intestine mucosa and absorbed into the small intestine mucosa (Fig. 3).

Figure 1: Structural features of the flavonoid subclasses.
Metabolisation and tissue distribution
In the case of high oral dosing too, flavonoids occur almost exclusively in conjugated form in the plasma (with the exception of epigallocatechin gallate). They are metabolically transformed already within the intestinal mucosa and in the liver prior to entry into the systemic circulation. As flavonoids already contain many reactive groups, above all conjugations (Phase II-Enzymes) such as methylations, glucuronidations and/or sulphations are important (Fig. 3). Both flavonoid-specific and inter-individual differences in the metabolite pattern have been described. The results of in vivo studies indicate intensive conjugation of the flavonoids taking place already in the small intestine. Non-conjugated flavonoidaglyca pass via the portal vein to the liver, where they may be conjugated (Fig. 3). In addition, quercetin and its metabolites have also been detected in the lymph of rats.
On the basis of the extensive metabolisation of flavonoids in the mammal organism described above, considerable quantities of flavonoids ingested orally are eliminated already through the small intestine mucosa membrane and via the gall so that only a variable proportion makes its way into the systemic circulation. Flavonoid conjugates that return to the intestinal lumen via the gall or through the intestinal mucosa can be partially reabsorbed (enterohepatic circulation). In addition to elimination via the gall, renal elimination is important for some flavonoids. For example 3 to 26 per cent of the doses of flavanones, isoflavones or flavonols are eliminated via the urine.
The tissue distribution of the flavonoids and their metabolites after oral ingestion is of particular interest for the transferability of results from in vitro or cell culture experiments as regards their possible relevance for the in vivo situation. After eleven weeks of oral application of quercetin (50 and 500 mg/kg body weight/day) in rats, by far the highest flavonol concentration was detected in the plasma. The lung, that showed the highest concentration of all the organs examined, contained an approximately six times lower flavonol concentration than the plasma. The lowest concentrations lying well below the concentrations described as effective in vitro were detected in the brain and in the white fatty tissue. In a long-term study (50 mg quercetin/ kg body weight/day for four weeks) with pigs, the highest flavonol concentrations were found in the kidney, colon, jejunum and liver. The lowest concentrations were detected in the M. long. dorsi and in the brain. The latter did not differ from the flavonol concentration determined in the plasma. Consequently, only the organs involved in metabolism or elimination offlavonols show higher flavonol concentrations than the plasma. These results indicate that especially in pigs no notable accumulation of flavonols takes place in the tissue even in the case of relatively long periods of application of quercetin. It should be noted here that by comparison with laboratory rodents and humans, pigs also appear to have a substantially higher capacity regarding the first pass elimination (Phase I and Phase II reactions) of flavonoids (own findings, not published), so that findings for laboratory rodents are only transferable to pigs subjectto substantial limitations.
On the basis of the extensive metabolisation of flavonoids in the mammal organism described above, considerable quantities of flavonoids ingested orally are eliminated already through the small intestine mucosa membrane and via the gall so that only a variable proportion makes its way into the systemic circulation. Flavonoid conjugates that return to the intestinal lumen via the gall or through the intestinal mucosa can be partially reabsorbed (enterohepatic circulation). In addition to elimination via the gall, renal elimination is important for some flavonoids. For example 3 to 26 per cent of the doses of flavanones, isoflavones or flavonols are eliminated via the urine.
The tissue distribution of the flavonoids and their metabolites after oral ingestion is of particular interest for the transferability of results from in vitro or cell culture experiments as regards their possible relevance for the in vivo situation. After eleven weeks of oral application of quercetin (50 and 500 mg/kg body weight/day) in rats, by far the highest flavonol concentration was detected in the plasma. The lung, that showed the highest concentration of all the organs examined, contained an approximately six times lower flavonol concentration than the plasma. The lowest concentrations lying well below the concentrations described as effective in vitro were detected in the brain and in the white fatty tissue. In a long-term study (50 mg quercetin/ kg body weight/day for four weeks) with pigs, the highest flavonol concentrations were found in the kidney, colon, jejunum and liver. The lowest concentrations were detected in the M. long. dorsi and in the brain. The latter did not differ from the flavonol concentration determined in the plasma. Consequently, only the organs involved in metabolism or elimination offlavonols show higher flavonol concentrations than the plasma. These results indicate that especially in pigs no notable accumulation of flavonols takes place in the tissue even in the case of relatively long periods of application of quercetin. It should be noted here that by comparison with laboratory rodents and humans, pigs also appear to have a substantially higher capacity regarding the first pass elimination (Phase I and Phase II reactions) of flavonoids (own findings, not published), so that findings for laboratory rodents are only transferable to pigs subjectto substantial limitations.

Figure 2: Mechanisms of the intestinal absorption of the flavonol quercetin
Can flavonoids subst itute Vit E supplements?
With regard to the action mechanisms offlavonoids, their high antioxidative potential is particular-Iy interesting. Savings of Vit E are among the aspects postulated here. Various authors do indeed show that at least in the test tube various flavonoids (including quercetin and catechin) can regenerate or save a-tocopherol (aTOC).
Lower meat quality is chiefly connected with the oxidation of lipids and fatty-soluble vitamins (for example Vit E). The use ofVit E (atocopheryl acetate) in quantities well exceeding the physiological demand can influence selected meat quality parameters such as, for example, pH value, meat colour and drip juice losses. In connection with possible alternatives to Vit E supplementing, the use of flavonoid-rich plant extracts (for example tea extracts) or industrial by-products (for example grape marc) in feeding productive livestock is currently a subject of intensive discussion. However, clear distinctions must be made between in vitro and in vivo studies, as in vivo the antioxidative effects of flavonoids are influenced by a variety of factors (for example local concentration, bioavailability, metabolisation). There are only a few results from in vivo studies by contrast with the many in vitro findings on the anti oxidative potential offlavonoids that deal with the interactions betweenflavonoids and Vit E. Due to the fact that most of these examinations were carried out on laboratory rodents and these results are regrettably frequently transferred to other species without any critical exploration, only the most important of these results are outlined below before corresponding studies in productive livestock are considered.
Lower meat quality is chiefly connected with the oxidation of lipids and fatty-soluble vitamins (for example Vit E). The use ofVit E (atocopheryl acetate) in quantities well exceeding the physiological demand can influence selected meat quality parameters such as, for example, pH value, meat colour and drip juice losses. In connection with possible alternatives to Vit E supplementing, the use of flavonoid-rich plant extracts (for example tea extracts) or industrial by-products (for example grape marc) in feeding productive livestock is currently a subject of intensive discussion. However, clear distinctions must be made between in vitro and in vivo studies, as in vivo the antioxidative effects of flavonoids are influenced by a variety of factors (for example local concentration, bioavailability, metabolisation). There are only a few results from in vivo studies by contrast with the many in vitro findings on the anti oxidative potential offlavonoids that deal with the interactions betweenflavonoids and Vit E. Due to the fact that most of these examinations were carried out on laboratory rodents and these results are regrettably frequently transferred to other species without any critical exploration, only the most important of these results are outlined below before corresponding studies in productive livestock are considered.
Studies on laboratory rodents
In Vit E-depleted rats, oral administration of quercetin (2 and 20 mg/day/animal over four weeks) led to higher aTOC concentrations in serum and liver. However, this could not be shown in "normal" Vit E supply (75 IU all-racatocopheryl acetate/kg diet). There was distinctly lower formation ofthiobarbituric acid reactive substances (TBARS), a (restrictedly useful) indicator for lipid peroxidation in both Vit E-depleted and normally supplied animals, whereby the effect was distinctly stronger after Vit E depletion. The authors therefore concluded that antioxidative effects of quercetin are manifest above all in cases of Vit E deficiencies. In a further study on rats higher aTOC concentrations appeared in the plasma and liver after four weeks of application of quercetin (2 g/kg diet), (-)-epicatechin (2 g/kg diet) and. (+)-catechin (2 g/kg diet) by comparison with the control group. As elevated supply of unsaturated fatty acids leads to an elevated turnover ofVit E in the organism' the influence of a flavonoid supplement (8 g/kg diet; quercetin:catechin 2:1) on a MUFA (mono unsaturated fatty acids) or PUFA (poly unsaturated fatty acids) enriched diet was examined in a further study. After four weeks the corresponding flavonoid groups showed higher alOC concentrations in the plasma and in the liver. By contrast, in another study no effect on the alOC concentration in the plasma and the tissues (liver, heart, muscle) of rats was shown following twelve weeks of quercetin application (5 g/kg diet). Even a Vit E depletion of the rats in this study did not lead to any "Vit Esaving" effect. Furthermore, the authors determined the malondialdehyde (MDA) concentration in the plasma and in the tissue. lhe analyses showed that the application of quercetin - even in the case of Vit E depletion - did not have any influence on the MDA concentration. The results offurther studies on rats lead one to suspect a direct antioxidative effect of flavonoids independent ofVitE.

Figure 3: Metabolisation of flavonoid·aglyca (sugar-free) in the small intestine mucous membrane and the liver.
Studies on productive livestock.
Several studies on hens looked at potential effects of supplementing grape marc (IT; contains high concentrations of (+ )-catechins, (-)-epicatechins, (-)-epicatechin- 3-0-Gallate and dimmer, timer and tetra mer procyanidins) and a-tocopheryl acetate on liver-aTOC concentration, body weight, nutrient digestibility and lipid peroxidation ofbreast and leg meat during storage. To summarise, the results show that neither supplementing with TI nor treatment with a-tocopheryl acetate influenced the health and zootechnical parameters. However, a comparison with the control group (95 mg Vit E/kg diet) showed that both supplements caused a lower lBARS concentration in breast and leg meat after storage at 4 °C. However, the addition of atocopheryl acetate was distinctly higher than the addition ofTI. TI supplementing resulted in higher aTOC concentrations in the liver by comparison with the control group, which the authors interpreted as a Vit E-saving effect. In a similar study with hens supplementing the diet with n concentrate (15, 30 or 60 g/kg diet) or with a-tocopheryl acetate (200 mg/kg diet) reduced the TBARS formation in stored breast meat to a similar extent. Feeding different quantities of tea catechins (TC; 50, 100, 200, 300 mg/ kg diet) to hens also led to higher aTOC concentrations and reduced TBARS formation in stored breast and leg meat by comparison with the control group (20-30 mg a-tocopheryl acetate kg/ diet), whereby a TC dose of 200 mg/kg diet demonstrated an effect compa rable to Vit E applications of 200 mg a-tocopheryl acetate/ kg diet. By contrast with this a further study did not reveal any influence on the a TOC concentration in chicken breast meat following application of a green tea extract (50 and 100 mg/kg diet) in combination with natural aTOC (50 or 100 mg/kg diet) by comparison with sole addition of aTOC (100 and 200 mg/kg diet) over six weeks.
In growing pigs administration of a green tea polyphenol extract (GTP; 10 and 100 mg GTP/kg body weight/day) with needs-covering Vit E supply (17 I.E./kg diet) over five weeks did not show any influence on the a TOC concentration in the plasma, liver, lung and back muscles, or on meat quality parameters and the antioxidative status. In a further study on pigs, supplementing with green tea catechins (GTC; 200 mg/kg diet) with needs-covering Vit E supply (45 mg a-tocopheryl acetate/kg diet) during final fattening did not have any effect on the a TOC concentration in the back muscles by comparison with the control group (55 mg a-tocopheryl acetate/ kg diet) either. With reference to the lipid peroxidation, however, after storage ofthe muscle meat for ten days under a modified atmosphere (40 per cent C02: 60 per cent 0 2) lower TBARS formation was found in the GTC group by comparison with the control group. In a study on Iberian pigs the application of a flavonoid or polyphenol-rich extract did not lead to any increase in the aTOC concentration or any reduction of lipid peroxidation in the M. long. dorsi. By contrast with the studies presented so far, in which the animals were supplied with Vit E covering needs or extending beyond the physiological demand, our own studies on growing pigs looked at possible Vit E savi ng effects of the flavonol quercetin (10 mg/kg live weight) with short Vit-E supply and additionally induced dietetic-conditioned oxidative stress (addition of 5 per cent fish oil to the diet). Without the addition offish oil the application of quercetin led to higher aTOC concentrations in the plasma and liver after four weeks. Consequently, a Vit Esaving effect of quercetin can be postulated in the case of short Vit E supply without additional "oxidative stress". The feeding of fish oil led to a further depletion of the plasma Vit E concentration and to a significant increase of the 8-isoprostaglandin F2a (8-iso-PGF2a) concentration, a marker for the in vivo lipid peroxidation. Under these conditions the application of quercetin admittedly had no effect on the plasma Vit E concentration, but it reduced the in vivo lipid peroxidation (reduced plasma concentration of 8-isoPGF2a).
In growing pigs administration of a green tea polyphenol extract (GTP; 10 and 100 mg GTP/kg body weight/day) with needs-covering Vit E supply (17 I.E./kg diet) over five weeks did not show any influence on the a TOC concentration in the plasma, liver, lung and back muscles, or on meat quality parameters and the antioxidative status. In a further study on pigs, supplementing with green tea catechins (GTC; 200 mg/kg diet) with needs-covering Vit E supply (45 mg a-tocopheryl acetate/kg diet) during final fattening did not have any effect on the a TOC concentration in the back muscles by comparison with the control group (55 mg a-tocopheryl acetate/ kg diet) either. With reference to the lipid peroxidation, however, after storage ofthe muscle meat for ten days under a modified atmosphere (40 per cent C02: 60 per cent 0 2) lower TBARS formation was found in the GTC group by comparison with the control group. In a study on Iberian pigs the application of a flavonoid or polyphenol-rich extract did not lead to any increase in the aTOC concentration or any reduction of lipid peroxidation in the M. long. dorsi. By contrast with the studies presented so far, in which the animals were supplied with Vit E covering needs or extending beyond the physiological demand, our own studies on growing pigs looked at possible Vit E savi ng effects of the flavonol quercetin (10 mg/kg live weight) with short Vit-E supply and additionally induced dietetic-conditioned oxidative stress (addition of 5 per cent fish oil to the diet). Without the addition offish oil the application of quercetin led to higher aTOC concentrations in the plasma and liver after four weeks. Consequently, a Vit Esaving effect of quercetin can be postulated in the case of short Vit E supply without additional "oxidative stress". The feeding of fish oil led to a further depletion of the plasma Vit E concentration and to a significant increase of the 8-isoprostaglandin F2a (8-iso-PGF2a) concentration, a marker for the in vivo lipid peroxidation. Under these conditions the application of quercetin admittedly had no effect on the plasma Vit E concentration, but it reduced the in vivo lipid peroxidation (reduced plasma concentration of 8-isoPGF2a).
Summary and conclusion
Vit E is the most important lipophilic antioxidant in the mammal organism and thanks to its hydrophobic character it is responsible above all for the protection of polyunsaturated fatty acids in cellular membrane structures. The antioxidative effect of the various natural and synthetic Vit-E derivates is well characterised here. Flavonoids that represent the main fraction of plant polyphenols occur in nearly all higher plants. However, natural contents in plant feedstuffs and in supplementsfluctuate considerably due to variable cultivation conditions, processing and treatment and storage. That is why standardising such products with regard to the content of "lead substances" is a fundamental and indispensable prerequisite for scientific assessment and serious marketing. Where possible alternatives to supplementing with Vit E as is commonly practised in productive livestock are concerned, discussion is focusing on the use of flavonoid-rich plant extracts (for example tea extracts) or industrial by-products (for example grape marc) as natural antioxidants in livestock feeding. In this connection current studies look at possible (Vit E-saving) or antioxidative effects of flavonoids in poultry and pigs. Although these studies show in some cases non-uniform and even contradictory results, it can be noted that none of the studies describe significant effects on live animals as regards zootechnical parameters such as feed intake, feed conversion, daily weight gains and growth performance. However, this applies for both supraphysiological doses of Vit E and for corresponding additions of supplements containing polyphenols. By contrast with this, in the case of shortVit E supply (however, this lies well below the doses customary in practice) and under conditions of manifest oxidative stress in the animal experiment, there are indications of a partial improvement of the Vit E status through polyphenols, but such findings will probably be virtually insignificant for farming practice as manufacturers provide considerable "safety margins" in supplementing with vitamins and active ingredients that by far exceed needs that ultimately lead to the productive animals being supplied well beyond what is necessary when mineral substance and vitamin supplements are used.
As regards the effects of supplementing rations with products containing polyphenols on meat quality parameters, it can be stated that in most of the studies positive effects are described above all on storage of meat. These can be compared "qualitatively" with the effects achieved by higher Vit E doses. In this connection, however, it must be notedthata "quantitative" comparison of various supplements is not possible, on the one hand due to insufficient characterisation and standardisation of most products with a polyphenol contentas regards their composition and the content of potentially active compounds, and on the other hand due to the lack of data regarding the "dose equivalence" of polyphenols and Vit E. In the opinion of the authors of this article, at the present time plant polyphenols are admittedly interesting "active ingredient candidates" in productive animals, but due to the few relevant studies available it is not justified to postulate overall that supplements containing polyphenols represent an equivalent replacement ofVit E. In this connection it should also be noted that both Vit E and polyphenols bring about many effects in the organism that are not based on their antioxidative action mechanisms and have not yet been sufficiently investigated.
As regards the effects of supplementing rations with products containing polyphenols on meat quality parameters, it can be stated that in most of the studies positive effects are described above all on storage of meat. These can be compared "qualitatively" with the effects achieved by higher Vit E doses. In this connection, however, it must be notedthata "quantitative" comparison of various supplements is not possible, on the one hand due to insufficient characterisation and standardisation of most products with a polyphenol contentas regards their composition and the content of potentially active compounds, and on the other hand due to the lack of data regarding the "dose equivalence" of polyphenols and Vit E. In the opinion of the authors of this article, at the present time plant polyphenols are admittedly interesting "active ingredient candidates" in productive animals, but due to the few relevant studies available it is not justified to postulate overall that supplements containing polyphenols represent an equivalent replacement ofVit E. In this connection it should also be noted that both Vit E and polyphenols bring about many effects in the organism that are not based on their antioxidative action mechanisms and have not yet been sufficiently investigated.








