



Poultry is Defenseless against Molds and Mycotoxins: Can We Help?
Aspergillus flavus is an extremely common soil fungus and an opportunistic pathogen of crops. The optimum temperature for A. flavus to grow is 37ºC, but fungal growth can be observed at temperatures ranging from 12 to 48ºC.A. flavus is also a pathogen of animals and insects. In humans, it is predominantly an opportunistic pathogen of immunosuppressed patients. A flavus is the main agent of acute and chronic invasive and granulomatous Aspergillus sinusitis. It is also an agent of otitis, keratitis, pulmonary and systemic infections in immunocompromised patients, cutaneous aspergillosis and aspergillosis in other vertebrates. Until recent years, the only drugs available to treat aspergillosis were amphotericin B and itraconazole, the latter in oral and intravenous formulations. Recently voriconazole, posaconazole and caspofungin have also been approved for the treatment of aspergillosis. Many A. flavus isolates produce aflatoxin B1, the most toxic and potent hepatocarcinogenic natural compound ever characterized. The discovery and isolation of aflatoxins was a result of investigations on the mysterious Turkey-X disease of 1960 which resulted in a loss of several thousand turkey poults in the United Kingdom. The cause of the enormous mortality in the turkey poults and of similar outbreaks in other farm animals could be linked to the use of moldy Brazilian peanut meal in the diet of the affected birds. The suspected toxic factor was found to be extractable by using chloroform. Its association with Aspergillus flavus (A. flavus) was established in 1961. In 1962, the name “aflatoxin”, using first letter from “Aspergillus” and the first 3 letters from “flavus” was proposed.
Actually, aflatoxins are a group of approximately 20 related fungal metabolites. The aflatoxins display potency of toxicity, carcinogenicity, mutagenicity in the order of AFB1> AFG1> AFB2> AFG2.
The Agency for Research on Cancer (IARC) classifies AFB1 as a class 1 carcinogen. Human and animals get exposed to aflatoxins by direct ingestion of aflatoxin-contaminated foods or ingestion of aflatoxins carried over from feed into milk and products like cheese and powdered milk as well as other animal tissues mainly as AFM1 and by inhalation of dust particles of aflatoxins especially AFB1 in contaminated foods in industries and factories. All species of animals are susceptible to aflatoxicosis and the susceptibility of individual animals to aflatoxicosis varies considerably depending on dose, duration of exposure, species, age, sex and nutrition. In animals, the aflatoxins cause liver damage, decreased milk production, reduced reproductively and suppressed immunity in animals consuming low dietary concentrations.
The aflatoxicosis syndrome in animals may also be characterized by vomiting, abdominal pain, pulmonary oedema, convulsions, coma, and death with cerebral edema and fatty involvement of the liver, kidneys, and heart. In dairy and beef cattle, the signs of acute toxicosis include anorexia, depression, dramatic drop in milk production, weight loss, lethargy, gastrointestinal dysfunctions such as ascites, icterus, abdominal pain, bloody diarrhea, decreased feed intake and efficiency; weight loss, jaundice, abortion, hepatoencephalopathy, blindness, walking in circles, ear twitching, frothy mouth, photosensitization, bleeding and death. In poultry, beside inappetance, weight loss, decreased egg production, leg and bone problems, poor pigmentation, fatty liver, kidney dysfunction, bruising and death, suppression to natural immunity and susceptibility to parasitic, bacterial and viral infections can occur.
UNIKE®PLUS efficacy against aflatoxins and fumonisins in broiler chicks
A total of 240 male Cobb day-old broilers were randomly allocated to 4 treatments with 6 replicates of 10 birds each. The experiment was 21 days long. Dietary treatments included:
| 1. | Mycotoxins: | feed contaminated with 1400 ppb of aflatoxins and 25 ppm of fumonisins |
| 2. | Mycotoxins+1,5 kg/t UNIKE®PLUS: | contaminated feed with 1,5 kg/t UNIKE®PLUS |
| 3. | Mycotoxins+ 2,5 kg/t UNIKE®PLUS: | contaminated feed with 2,5 kg/t UNIKE®PLUS |
| 4. | Mycotoxins+ 5 kg/t UNIKE®PLUS: | contaminated feed with 5 kg/t UNIKE®PLUS |
Feed and water were offered ad libitum during the entire experimental period as typical in battery managed birds except on weighing days when the feed was removed for 6 hours prior to weighing. The feed was contaminated with mycotoxin culture material (75% aflatoxin B1, 24% aflatoxin G1, 1% aflatoxin B2+G2 and 94% fumonisin B1, 6% fumonisin B2).
Parameters measured were:
- individual body weight
- feed intake and feed conversion ratio (FCR)
- individual relative liver weight (expressed as percentage)
- blood parameters such as total plasma proteins, cholesterol and albumin
The data was analysed by analysis of variance (ANOVA) and the Bonferroni test (p<0.05) was used to compare the means.
At 21 days of age, birds fed diets with mycotoxins presented the lowest body weight as compared to other groups (Fig. 1). The body weight in groups with UNIKE®PLUS was higher and at the inclusion rate of 2,5 and 5 kg/t, the difference with the contaminated group was significant.
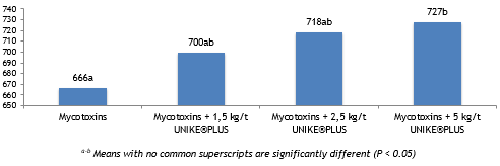
Fig. 1. The effect of different dosages of UNIKE®PLUS on body weight of young chicks
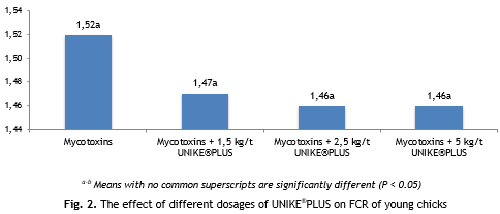
No statistically significant difference between treatments for FCR was observed (Fig. 2). However, there was a substantial numerical difference that was dependent on UNIKE®PLUS dose.
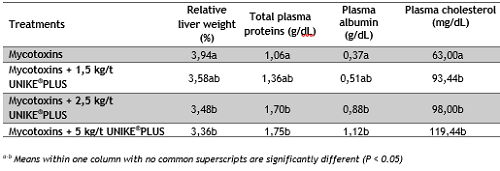
Mycotoxins, especially aflatoxins, damage liver and impact protein synthesis as well as fat metabolism. Broilers fed on the diet containing mycotoxins had the highest liver weight and the lowest total plasma protein, albumin and cholesterol levels (Table 1). When added to the contaminated diet, UNIKE®PLUS had a clear dose effect on liver status and several blood parameters. Birds receiving UNIKE®PLUS at levels of 2,5 and 5 kg/t presented significantly improved values in lower relative liver weight and higher blood serum
Table. 1 Organ status and blood parameters
Conclusion
High levels of aflatoxins in combination with fumonisins can significantly affect animal growth performance and health. The use of UNIKE®PLUS can successfully reduce the negative effects of mycotoxins. In this experiment, broilers fed contaminated diet showed a clear dose response with the inclusion of UNIKE®PLUS. Thus, UNIKE®PLUS is an effective tool in reducing the negative impact of mycotoxins on animal growth performance and health.
UNIKE®PLUS works strictly in vivo and will not counteract or mask mycotoxin in stored feed or raw ingredients. UNIKE®PLUS deactivates the toxins directly in the gastrointestinal tract of animals, based either on adsorption of those mycotoxins with suitably located polar functional groups, or biological degradation (bio-inactivation). UNIKE®PLUS and all NUTRIAD specially developed feed additives protect animals from mycotoxicoses by adsorption, bio-inactivation, organ, immune and antioxidant system support and represent an optimal solution for mycotoxin management for farm animals. NUTRIAD mycotoxin deactivators do not interact with vitamins, minerals or medication in vivo. Above mentioned study shows that application of effective mycotoxin deactivator offers an opportunity to significantly improve animal health, performance and productivity impaired by mycotoxins. Depending on the target performance different mycotoxins can be less or more problematic. Therefore, using different products for different animal groups become a rational trend.
Radka Borutova, DVM, PhD, Nutriad International, Belgium








