



ProBe-Bac®, a new therapeutic approach for bacterial infection
Antibiotics are not the right solution to Salmonella problemThe International Association for Food Protection have addressed the issues on how the contamination of poultry with Salmonella remains one of the crucial concerns in food safety during the annual meeting on August 2022. Different serotypes of Salmonella such as Enteritidis, Gallinarum, Pullorum and Typhimurium are accountable for numerous cases associated to poultry. Study has shown that Salmonella-contaminated animal products bring about 3% of the bacterial foodborne disease globally, with an approximately 115 million infections and 370,000 deaths per year (Abd El-Hack et al., 2021; El-Saadony et al., 2022; Qin et al., 2022). Meanwhile, government, research institutes, and industries are undertaking multiple efforts to better understand and control Salmonella contamination, and to develop effective interventions, which in the long run, improving public health.
Antibiotics are not the right solution to Salmonella problem
Due to the incidence of antimicrobial resistance (AMR) in some specific serotypes of Salmonella, it is becoming the focus issue nowadays. The unrestricted application of antibiotics in animal production and treatment of human and animal diseases induced its resistance to one or more antibiotics. Meanwhile, the World Health Organization (WHO) warned the public on the serious threat of using antibiotics in livestock feed. They emphasized the accumulation of antimicrobials in the body of the animal once it is incorporated in their feed, which in return harmfully affects human health and immune system through food chain.
The National Antimicrobial Resistance Monitoring System (NARMS) for enteric bacteria is a US public health surveillance system that tracks changes in AMR in foodborne and other enteric bacteria. Data from NARMS 2019 Integrated Summary in combination with the Centers for Disease Control and Prevention (CDC), U.S. Food and Drug Administration (FDA), and U.S. Department of Agriculture (USDA) provides information on resistance patterns found in bacteria isolated from humans, animals, raw meats from slaughter facilities, and poultry products. The NARMS reported Salmonella infection cases with an estimated 1.35 million illnesses and 26,500 hospitalizations every year (CIDRAP, 2022).
Increasing multi-drug resistance of Salmonella
The NARMS report shows the increasing resistance of Salmonella to ciprofloxacin (one of the three antibiotics in treating severe Salmonella infection). In connection to this, data from 2018 to 2019 shows Salmonella with decreased susceptibility to ciprofloxacin increased from 9% to11% in humans, from 20% to 30% in chicken product samples, and from 26% to 32% in chicken cecal content samples (US FDA, 2022). Multi-drug resistance in Salmonella isolated between 2018-2019 from chicken product samples showed an increase from 22% to 29%. Ceftriaxone resistance in human isolates remained below 4% in 2019 as similar to previous years. In 2019, multi-drug resistant Salmonella remained stable at around 10% which accounts for 26% of multi-drug resistance Salmonella. Consequently, antibiotic resistance of Salmonella isolated from cecal contents at slaughter facilities in USA showed that 64% resistance accounts for 9 out of 13 tested antibiotics, while 14% and 22% were sensitive and intermediates, respectively (NARMS, 2019)
Bacteriophages: safe and sustainable alternative to antimicrobials
A bacteriophage is a natural antibacterial entity that has been used before antibiotics were even discovered. The efficacy of antibiotics was reduced, as antibiotic-resistant bacteria emerged. Subsequently, bacteriophages have attracted much attention as a potential alternative solution.
Bacteriophage infects bacteria by attaching to the bacterial cell membrane and injecting its genetic information into the bacterial host. Afterwards, through the bacterial host machinery, bacteriophage proliferates that lead to lysis of the bacterial cell by either lytic or lysogenic cycle. Bacteriophages have high specificity to the target hosts which can be non-selective in case of antibiotics.
The US FDA has verified the safety of using bacteriophages, and also approved as GRAS (Generally Recognized as Safe) substance. The potential for bacteriophage application in livestock is attracting more attention by its advantages. Hence, Pathway Intermediates, together with Optipharm, its affiliate company leading in the field of animal diagnosis and biomedical research, are exerting effort for the development and commercialization of a new bacteriophage solution called ProBe-Bac.
ProBe-Bac is the latest bacteriophages solution of Pathway Intermediates. It is a cocktail product which contains a powerful mixture of bacteriophages precisely selected against specific disease. The newly developed ProBe-Bac has improved stability and coverage which maximizes the efficiency in eliminating bacterial pathogens. Meanwhile, ProBe-Bac PE is one version of the product specifically for poultry which targets diseases like fowl typhoid, diarrhea, and salmonellosis. The bacteriophages in ProBe-Bac would settle in the animal intestine upon ingestion, which selectively kills pathogenic bacteria in a specific-manner, improves intestinal environment, and boosts feed efficiency.
Effects of ProBe-Bac PE on growth performance and microbial population of broiler
A recent study investigated the beneficial effects of ProBe-Bac PE on growth performance and microbial population in broilers. Results has been presented and published recently in Scientific Reports Journal (Sarrami et al., 2022). The investigation was carried out with 1,200 day-old chicks (Ross 308). Treatments include CON (basal diet), 0.05% PB (CON + 0.05% ProBe-Bac PE), 0.10% PB (CON + 0.10% ProBe-Bac PE), 0.15% PB (CON + 0.15% ProBe-Bac PE), and 0.03% ATB (CON + 0.03% Colistin).
The average daily gain (ADG) and feed conversion ratio (FCR) significantly improved for the animal group that received different inclusion levels of ProBe-Bac PE (Figure 1). In finisher phase, 0.05% PB, 0.10% PB, and 0.15% PB treatments had significantly higher ADG than 0.03% ATB treatment (P<0.01), whereas FCR of 0.10% PB, and 0.15% PB treatments was significantly lower compared to 0.03% ATB treatment (P<0.01). Dietary inclusion of ProBe-Bac PE increased the body weight gain and reduced the FCR compared to the control and Colistin-fed groups during finisher and overall period (P<0.05).
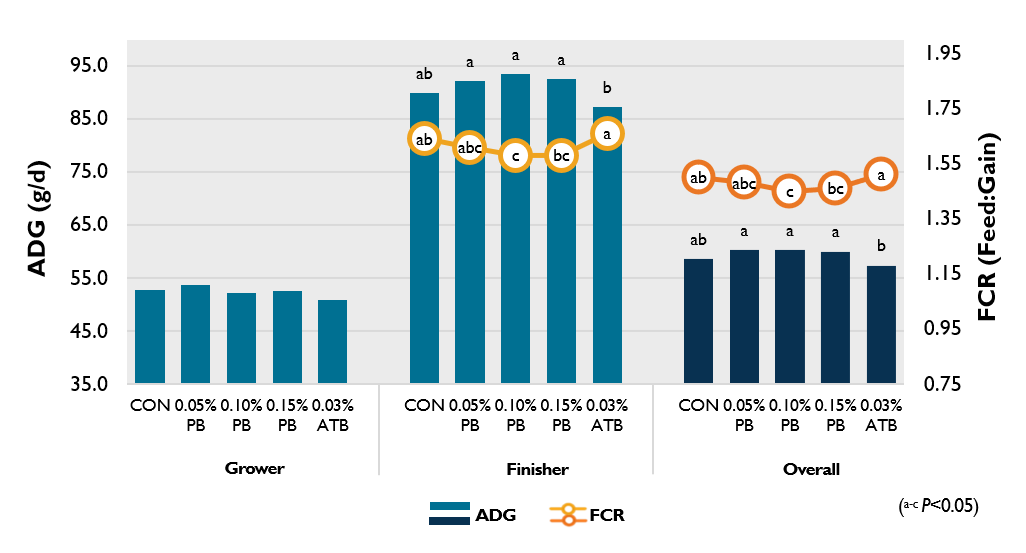
Figure 1. Effect of ProBe-Bac PE supplementation on growth performance of broilers.
Dietary inclusion of ProBe-Bac PE increased Lactobacillus and reduced the coliform bacteria counts in the ileal contents (P<0.05) compared to the control group (Figure 2). Overall, these results indicate that bacteria might developed defense mechanisms and resistance against antibiotics, while ProBe-Bac PE, as the latest bacteriophage solution which specifically recognize and attack target bacterial host, could be suggested as an alternative to antibiotic contributing growth promotion in broilers.
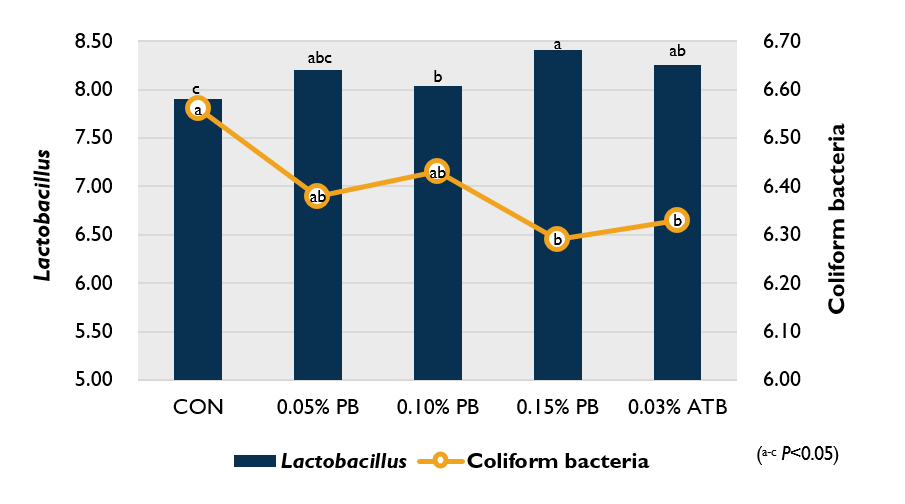
Figure 2. Effect of ProBe-Bac PE supplementation on microbial population of the ileal contents (log10 CFU/g) of broilers.
Conclusion
Results of the current study showed that supplementation of ProBe-Bac PE improved growth performance which was even better than antibiotic treatment. Moreover, ProBe-Bac PE supplementation could decrease pathogenic bacteria, thus, improving the intestinal microflora of the broilers. Collectively, these results indicate that ProBe-Bac PE could be considered a promising alternative to growth-promoting antibiotics and intestinal microflora improvement in broilers.
For more information, please contact your Pathway Intermediates representative.








