



Public Health Risk-Based Inspection System for Processing and Slaughter
By the USDA, FSIS. The US Food Safety and Inspection Service (FSIS) is proposing a Public Health Risk-Based Inspection System (PHRBIS) for all processing and slaughter establishments.The components of the proposed PHRBIS are science-based and are being designed with input from stakeholder groups and expert peer review.
The proposed PHRBIS would be developed within the regulatory framework of current FSIS inspection activities (ie, verification of HACCP, SSOPs, SPS and other regulatory requirements), but would provide more of a focus on process steps that are vulnerable to microbial contamination if there is a loss of process control. In addition, FSIS would use the PHRBIS to focus its flexible inspection resources, such as Food Safety Assessments (FSA) and in-depth verification testing (IVT), on establishments with a high risk for microbial contamination.
The purpose of the PHRBIS is to focus FSIS’ inspection resources on those areas of greatest food safety risk, improving the Agency’s ability to protect public health while maintaining the levels of inspection at all Federally-inspected establishments required under the Meat Inspection Act, Poultry Products Inspection Act, and Egg Products Inspection Act. An important aspect of implementing the proposed PHRBIS is to ensure that the basis for decisions are clearly delineated, transparent, and scientifically-driven (including being data-driven) whenever possible and appropriate.
The proposed PHRBIS, which is described in this report, evolved from FSIS’ earlier work on developing a Risk-Based Inspection (RBI) algorithm to rank processing establishments. As can be seen from this report, the system currently under consideration addresses many of the concerns expressed by the USDA Office of the Inspector General (OIG, 2007), industry, and consumer groups regarding the earlier RBI algorithm.
Foodborne disease is a public health concern for the US population. The most commonly recognized foodborne infections in the United States are those caused by the bacteria Campylobacter, Salmonella, and Escherichia coli O157:H7 (E. coli O157:H7), and by a group of viruses known as Norwalk-like viruses (CDC 2007). Norwalk-like viruses cause an estimated 66 percent of foodborne illness in the US (CDC 1999). FSIS’ public health goals focus on reducing Salmonella, Escherichia coli O157:H7 (O157:H7), and Listeria monocytogenes (Lm).
FSIS estimates that approximately 60 per cent of the Salmonella foodborne illnesses due to FSIS regulated products in 2006 are attributable to poultry products. In 2006, FSIS Salmonella verification testing found 11.4 per cent of positive samples, down from a high of 16.3 per cent in 2005. In addition, of the 184 sets completed in 2006 at broiler establishments, 88.6 per cent met the Salmonella performance standard, up from 81.3 per cent in CY 2005.
In order to meet the Healthy People 2020 goal of 6.8 Salmonella cases per 100,000 persons, the Agency has set an objective of 90 per cent of broiler establishments to be in Salmonella Category 1 by 2010. In FY 2006, 49 percent of establishments were in Salmonella Category 1. In FY 2007, that number had increased to 73 per cent. The proposed PHRBIS will be an essential tool for the Agency to meet its proposed Salmonella performance objective.
FSIS estimates that approximately 60 per cent of the E. coli O157:H7 foodborne illnesses due to FSIS-regulated products in FY 2006 are attributable to ground beef. In FY 2006, E. coli O157:H7 FSIS verification testing found 0.17 per cent of positive samples, down from 0.71 per cent in FY 2000. That number can also be calculated adjusting for volume to make it more representative of potential exposure. When volume adjusted, the FY 2006 value is 0.40 per cent; in FY 07 the volume adjusted percentage was 0.28 per cent. The Healthy People 2010 goal for E. coli O157:H7 is one case per 100,000 persons. That translates to a volume adjusted objective of 0.18 percent positives in FSIS’ ground beef sampling programme.
FSIS estimates that approximately 71.6 per cent of the Listeria monocytogenes foodborne illnesses due to FSIS-regulated products in 2006 are attributable to ready-to-eat (RTE) products. In 2006, FSIS Listeria monocytogenes verification testing of RTE product found 0.61 per cent of positive samples, down from 1.45 per cent in 2000. The Healthy People 2010 goal for Listeria monocytogenes is 0.24 cases per 100,000 persons. In order to meet that goal, the Agency has set a volume weighted performance objective of 0.28 per cent in RTE products by 2010.
This proposed system is being developed with the goal of decreasing foodborne pathogens and moving FSIS toward meeting its pubic health goals and objectives. FSIS believes that the proposed PHRBIS will be better able to protect public health by focusing and integrating our regulatory authority on establishments and process points within slaughter and processing establishments at which control of microbial contamination can have the greatest impact. Similarly, it believes that the incorporation of performance standards in the PHRBIS will incentivize industry to decrease the amount of microbial contamination that occurs.
The Agency has learned from its experience with Hazard Analysis and Critical Control Points (HACCP) and food contamination events that to better protect public health it must bolster its inspection forces’ ability to link and respond to noncompliances within establishments. In addition, the Agency also learned that its inspectors must verify not only Critical Control Points of an establishment's overall food system, but also verify the execution of the decisions made by the establishment in the Hazard Analysis, particularly prerequisite programmes.
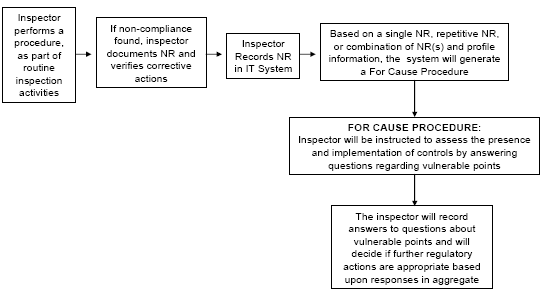
Inspectors will be prompted by the new IT system to focus their activities on vulnerable points in the process.
As described in this report, the Agency is proposing data driven and science-based methods for allocating inspection activities both across and within establishments to meet those needs. By working within its existing regulatory framework, the PHRBIS will focus FSIS inspection resources on those establishments and points within slaughter and processing that can have the greatest impact upon the microbial contamination of products. This is essential because FSIS cannot test all finished product at an establishment, and must have a means of ensuring that process control is consistently maintained. To support its proposed PHRBIS, FSIS is redesigning its Information Technology (IT) System to facilitate better collection of inspection data regarding establishments. The new IT system will help FSIS inspectors and headquarters personnel link and respond to inspection activity findings within establishments.
This report outlines the elements of the PHRBIS for processing and slaughter establishments, and discusses the scientific basis for those elements. It begins with a discussion of the proposed approach for focusing inspection activities within an establishment, followed by the approach for allocating flexible inspection resources across establishments. Each of those approaches has been designed with the goal of identifying and preventing potential public health hazards in establishments before they reach the consumer. Next, the Agency’s evaluation plan for the proposed PHRBIS are discussed in the report. Appendices supporting and detailing the sections include: attribution and performance measures, inspection prompt tables, scientific literature reviews, data sources, and data analyses.
Further Reading
|
|
- You can view the full report by clicking here. |
January 2008








